Lewis Structure Of H2o
The Lewis structure of hydrogen and 2 oxygen atoms shows a total of eight valence electrons participate in the bond formation to form a single triatomic H2O molecule.
Here, we need to understand how the Lewis structure is drawn for the H2O molecule:
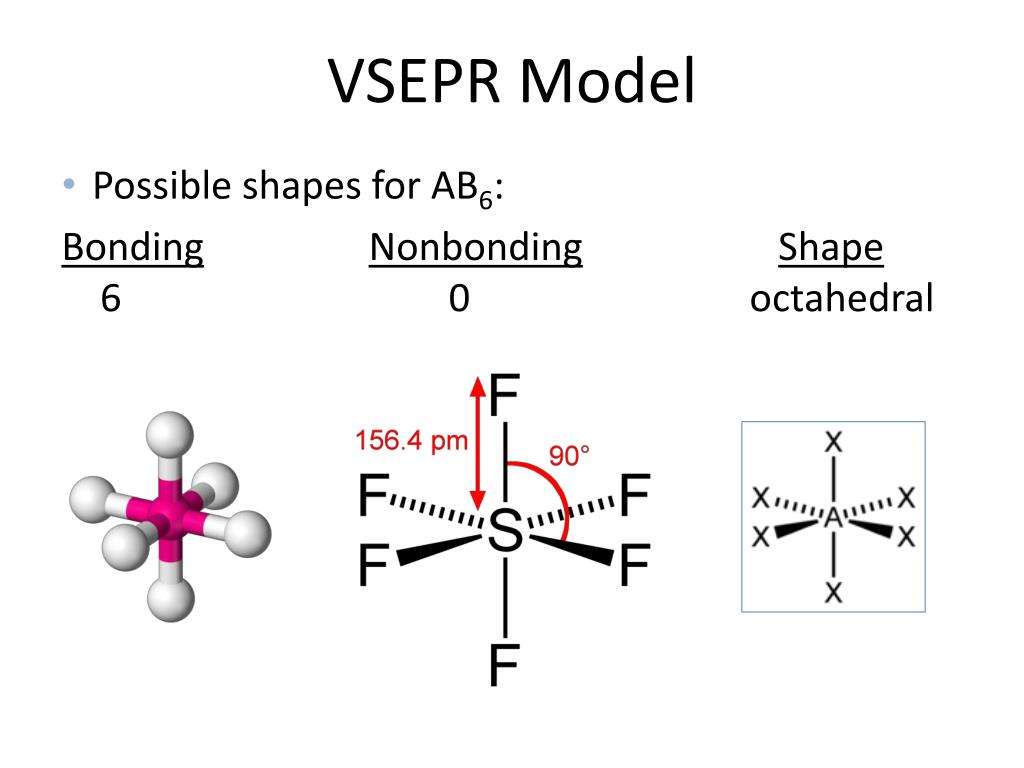
What Is The Connection Between The Number Of Electron Domains And The Electron Geometry
3.9/5Electron geometrygeometricELECTRONnumber of electronElectron domain geometriesnumber of electronbonding
Also, how electronic shape is affected by the number of electron domains?
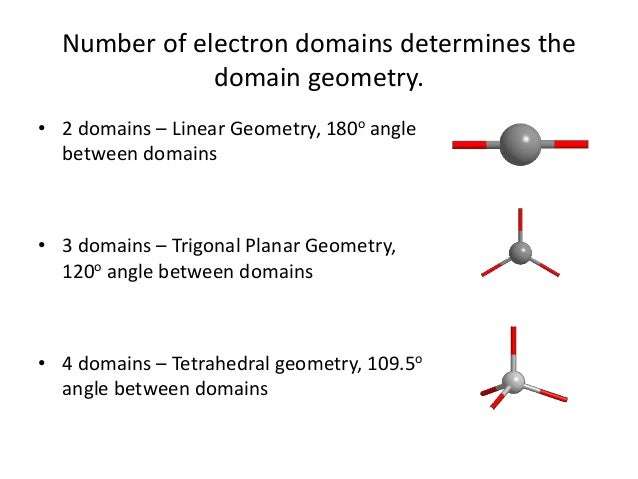
Electronic shape and name is drastically influenced by the number of electrons domains. Increasing electron domains, means changing name and shape. For example, two electron domain would mean linear and 180 degree shape. While 3 electron domains means trigonal planar and 120 degrees.
Also, what is the electron domain geometry? Central Atom with One or More Lone Pairs. The total number of electron pairs, both bonding pairs and lone pairs, leads to what is called the electron domain geometry. Electron domain geometries are one of the five learned so far: linear, trigonal planar, tetrahedral, trigonal bipyramidal, or octahedral.
Moreover, what is the electron pair geometry of sncl3?
SOLUTION The Lewis structure for the SnCl3– ion is: The central Sn atom is surrounded by one nonbonding electron pair and three single bonds. Thus, the electron–pair geometry is tetrahedral with three of the corners occupied by the bonding pairs of electrons.
What is the relationship between geometric shape and electron pairs?
Final Step Check The Stability Of Lewis Structure By Calculating The Formal Charge On Each Atom
Now, you have come to the final step and here you have to check the formal charge on oxygen atom as well as each hydrogen atom .
For that, you need to remember the formula of formal charge
Formal charge = Valence electrons Nonbonding electrons /2
- For Hydrogen: Valence electron = 1 Nonbonding electrons = 0
- Valence electron = 6 Nonbonding electrons = 4
Don’t Miss: Angle Addition Worksheets
Hybridization Of H2o Molecule
The bond between each oxygen and hydrogen atom in a water molecule is sigma with no pi bonds.
As we know, sigma bonds are the strongest covalent bonds. As a result, there is high stability between the oxygen and the hydrogen atom.
It is the two lone pairs on the oxygen atom which makes all the difference. The hybridization of a water molecule is sp3, where its oxygen has been hybridized.
According to the diagram, it can be analyzed that the single oxygen atom in the water molecule has one 2s orbital and three 2p orbitals. These four altogether leads to the formation of four sp3 hybridized orbitals.
It leads to the formation of the tetrahedral bent geometry, where overall H2O molecule shows 25% characteristics of s and 75% characteristics of the p orbital.
It can further be explained with the help of a molecular orbital diagram of the H2O molecule.
The 2s orbital and three 2p orbitals of the oxygen atom forms four new hybrid orbitals which further bonds by undergoing overlapping with the 1s orbital of the hydrogen atoms.
The Molecular Geometrical Of The H2o Lewis Structure For The Molecular Cluster
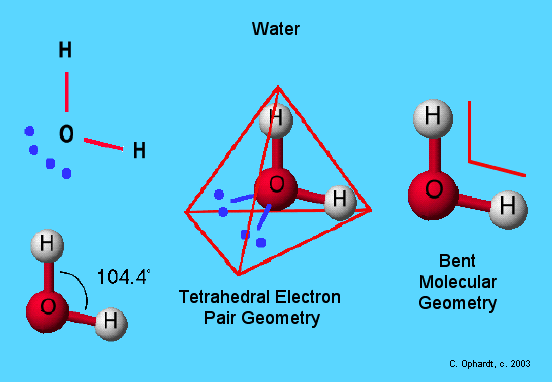
The bond angle between helium, oxygen, and hydrogen atoms is 104.5°. The geometrical configuration of a single H2O molecule is twisted, as can be seen from this. The water molecule is nonlinear, with an above said angle.
Its demonstrated by the Valence Shell Electron Pair Repulsion principle, which explains why the bond angle on the oxygen atom is limited to 104.5° despite the presence of two pairs of lone electrons.
A bent-shaped molecules optimal bond angle is 109.5°. Because each O-H bond are polar covalent in nature, with more positive end at H as compare with O end. This induce H2O molecule higher dipole moment.
When all of the valence electrons around the atom are not paired, a lone pair occurs, according to the Lewis structure.
The oxygen atom in the H2O molecule, which has two lone pairs, is similar.
Due to the lone pair-lone pair repulsion, which is greater than the bond pair-bond pair and lone pair-bond pair repulsion, these lone pairs skew the bond angle.The bond angle reduces as the lone pair increases. Since the oxygen atom has two lone pairs, the bond angle is reduced to 104.5°.
Read Also: Paris Jackson Mom And Dad
Select The Center Atom
While selecting the center atom, always put the least electronegative atom at the center.
Here in the H2O molecule, if we compare the hydrogen atom and oxygen atom , then hydrogen is less electronegative than oxygen. But as per the rule, we have to keep hydrogen outside.
So, oxygen should be placed in the center and the remaining 2 hydrogen atoms will surround it.
How Many Pairs Of Electrons Are In Chloride
Explanation: An isolated chlorine ATOM has 7 valence electrons. And thus the parent dichlorine molecule tends to be an OXIDANT, viz And because the resultant chloride anion has FOUR SUCH LONE PAIRS, instead of the SEVEN electrons associated with the NEUTRAL atom, chloride has a FORMAL NEGATIVE CHARGE.
Read Also: Michael Jackson Kids Biological
Difference Between Electron Geometry And Molecular Geometry
The name of the geometry of the electron pair/groups/domains on the central atom, whether they are bonding electrons or non-bonding electrons, is referred to as electron geometry. It enables us to understand how different electron groups in a molecule are arranged. Molecular geometry, on the other hand, is the three-dimensional structure of atoms in a molecule that determines its shape. It aids in comprehending the entire atom and its arrangement.
If all of the electron groups are bonded, and there are no lone pairs, the electron geometry and molecular geometry are the same.
Key Takeaways: Electron Domain
- An atom’s electron domain is the number of lone pairs or chemical bond locations that surround it. It represents the number of locations expected to contain electrons.
- Electron repulsion is not the only factor that affects molecular geometry. Electrons are attracted to positively charged nuclei. The nuclei, in turn, repel each other.
Also Check: Paris Jackson’s Father
The Geometrical Structure Of The H2o Molecule
The bond angle among hydrogen-oxygen-hydrogen atoms is 104.5°. From this, it can be understood that the geometrical structure of a single H2O molecule is bent.
It is explained with the help of the Valence Shell Electron Pair Repulsion theory, which says why irrespective of having two pairs of lone electrons on the oxygen atom the bond angle is reduced to 104.5°.
The ideal bond angle for a bent-shaped molecule is 109.5°.
According to the Lewis structure, there exists lone pair when all the valence electrons around the atom are not paired.
Similar is a case of the oxygen atom in the H2O molecule, where two lone pairs exist.
These lone pairs distort the bond angle due to the lone pair-lone pair, which is more than the bond pair-bond pair and lone pair-bond pair repulsion.
When the lone pair increases, the bond angle decreases. As there are two lone pairs on the oxygen atom, it reduces the bond angle to 104.5°.
S02 Is Polar Or Nonpolar
Sulfur dioxide is polar in nature. The difference in electronegativity between sulfur and oxygen atoms creates polarity in the molecule. Oxygen has a greater electronegative potential than sulfur. Therefore, oxygen exerts more pull on the covalent bonds in sulfur dioxide. The portion of the molecule that has both oxygen atoms on it is slightly negatively charged.
Whereas, the portion which has the sulfur atom has a slightly positive charge. This makes SO2 a polar molecule like H2S. In addition, the unbonded electrons on the sulfur and oxygen create repulsion between atoms.
This is another cause of the polarity of the sulfur dioxide molecule.
Read Also: Beth Thomas Biological Father
Is Water Polar Or Nonpolar
Water is a polar compound because it has an unequal sharing of electrons. In H2O molecules, the two hydrogen atoms combine with one oxygen atom. Among two out of the four electrons in oxygen, it forms the strong bond in water that concludes there is an unequal sharing of electrons as two of the atoms remain unused. As the oxygen atom has a stronger attractive force that draws more atoms towards it which also causes a charge imbalance within the molecule.
Electronegativity of H2O
As we know, Electronegativity explains the degree to which an atom attracts electrons in a chemical bond. The difference in electronegativity of two atoms determines the chemical bond.
In an H2O molecule, Hydrogen has an electronegativity of 2, while oxygen has an electronegativity of 3.5. So basically their difference is 1.5 which means water is a polar covalent molecule. This also concludes that the electrons are drawn significantly towards the more electronegative element.
The dipole moment of H2O
Hydrogen Peroxide Lewis Structure Molecular Geometry Polar Or Non
Home > Chemistry Article> H2O2 lewis structure and its molecular geometry
Hydrogen peroxide is slightly more viscous than water with the chemical formula H2O2. It is unstable and decomposable in the presence of light. It can found in the Human body also.
In this article, we will discuss the Hydrogen peroxide lewis structure, its molecular geometry/shape, bond angle, hybridization, Is H2O2 polar or nonpolar, etc.
In pure form, H2O2 is a colorless liquid with a bitter taste. It is the simplest peroxide with an oxygen-oxygen single bond. H2O2 becomes very dangerous when it is heated to boiling temperature even it can explode and cause severe burns.
Useful properties of Hydrogen peroxide
- It is a strong oxidizer agent.
- It has a bitter taste.
- H2O2 is nonflammable.
Recommended Reading: Write The Segment Addition Postulate For The Points Described
When You Draw A Lewis Structure For H2o How Many Electron Groups Domains Are Around The Central Atom
four electron pairsWater has four electron pairs and the coordination geometry of oxygen is based upon a tetrahedral arrangement of electron pairs.VSEPR calculation for water, OH. 2.
| Lewis structure: |
| 8 |
|---|
How do you count domains?
Count the total number of electron domains. Use the angular arrangement of the chemical bonds between the atoms to determine the molecular geometry. Keep in mind, multiple bonds count as one electron domain. In other words, a double bond is one domain, not two.
How many electron domains does f have?
One Domain
How many lone pairs of electrons are present in H2O?
two lone pairsAB2E2: Water, H2O A water molecule consists of two bonding pairs and two lone pairs .
H2co Lewis Structure Molecular Geometry Hybridization And Mo Diagram
It is an organic compound with the molecular formula of H2CO and is classified as an aldehyde. Aldehydes are chemicals having the functional group -HCO- in their molecules and formaldehyde is the lowest member of this group with a single carbon atom.
It shows unique physical and chemical properties due to its molecular structure and bonding. Molecular formaldehyde is a colorless, toxic gas with an irritating and pungent smell. In its solid form, it can exist either as a trimer or a polymeric form is known as Paraformaldehyde.
Formaldehyde is usually stored in aqueous solutions known as formalin. This chemical, due to its high reactivity is also one of the most important building blocks of synthetic chemistry.
One of the biggest clues to this versatility in the physical state as well as the reactivity of formaldehyde lies in the nature and structure of its chemical bonds. A good starting point in understanding them will be the Lewis structure.
Recommended Reading: Lesson 4.5 Practice B Geometry Answers
Geometrical Or Molecular Shape:
The geometrical structure of any molecule has great influences on the polarity nature. As the molecular geometry of H2O2 is bent and electron geometry is tetrahedral it shows that dipoles cannot be canceled out because they lie in two different planes.
Also, lone pair present in the Hydrogen peroxide lewis structure cause uneven charge distribution around atoms that makes the H2O2 structure Non-planar.
So, all these factors show Why H2O2 is a Polar molecule.
Connect Hydrogen And Oxygen With A Single Bond
After placing hydrogen and oxygen, now its time to connect them with a single bond for further drawing the H2O2 lewis dot structure.
Now look at the above structure, we use three single bonds to connect oxygen and hydrogen. As we know one single bond contains two electrons.
Hence, three single bonds in the above structure mean we use 6 electrons from total valence electrons.
= 8 valence electrons
Now we are left with 8 valence electrons.
You May Like: Geometry Chapter 7 Quiz 1 Answer Key
H2co Molecular Orbital Diagram
So far, we have covered many structural aspects of the formaldehyde molecule by using hybrid orbitals and VSEPR theory. In addition, we have a molecular orbital theory , the most in-depth analysis of chemical bonding using quantum mechanical properties.
MOT describes electron density and distribution in molecules similar to atomic orbitals in atoms. It uses a wavefunction to describe the behavior of valence electrons, molecular orbitals are filled up in the same way as atomic orbitals and the total no. of molecular orbitals formed = no. of combining atomic orbitals.
The molecular orbital diagram represents energy on the Y axis, with bonding molecular orbitals lower in energy than anti-bonding orbital. A bonding molecular orbital represents electron density in between the two nuclei where attraction overcomes repulsion.
An anti-bonding orbital represents the region between two nuclei where repulsion is greater than attraction and no bond is formed. Non-bonding orbital represents atomic orbitals which essentially remain localized on a single atom and do not take part in bond formation.
When there are more than two atoms involved in bond formation, an approximation method called a linear combination of atomic orbitals is used, like in formaldehyde.
MOT helps us determine electrostatic potential maps, excited state properties, probable molecular spectra, and predict the reactivity of the molecule.
H2s Molecular Geometry Or Shape
To determine the electron and molecular geometry of H2S, its lewis structure, and VSEPR theory play an important role.
Electron geometry considers all electrons as well lone pairs to determine the geometry of H2S whereas Molecular geometry only considers bonded atoms of the molecules to determine the shape of H2S.
Also Check: Geography Movement Definition
H3o+ Lewis Structure Geometry Hybridization And Mo Diagram
The cation H3O+ is known as the hydronium ion. If we see the nomenclature of hydronium ion, we get to know that according to the IUPAC nomenclature, hydronium ion can be referred to as oxonium.
Oxonium is a generalized name for all trivalent oxygen cations, so the use of the name hydronium is necessary to identify hydronium ions particularly.
The molar mass of the hydronium ion is 19.02 g/mol.
The production of this ion is during the protonation of water
H2O + H+ > H3O+
This ion is used in determining the pH of water. As water dissociates into OH- and H3O+ and we can check the pH of the water using a particular process.
The hydronium ion is used in various reactions and the production of different compounds. Both organic and inorganic chemistry includes hydronium ion to a large extent.
But before reading the use of this ion in different reactions, we must have knowledge about the basics of this ion, like, lewis structure, geometry, etc.
Knowing these basics will deepen our knowledge about this ion more. We should always try to know the background of any compound before studying any reaction regarding it.
So lets dig deep and know about some interesting information about hydronium ion!
What Is The Octet Rule Law
The states that an atom should have a maximum of eight valence electrons. Furthermore, in the Lewis structure, these eight electrons are drawn just around the atoms sign.
Two valence electrons are of short supply in oxygen. The two hydrogen atoms, on the other hand, have a limit of two valence electrons missing.
The Lewis structure of H2O is drawn in such a way that each atoms deficiency is satisfied.
You May Like: Beth Thomas Now 2020
How To Draw The Lewis Structure Of H2o
Follow the steps given below to draw the lewis dot structure of H2O easily.
Hydrogen has 1 electron in its outermost shell whereas oxygen has 6. In order to satisfy the octet rule, the oxygen atom needs 2 electrons and 2 hydrogen atoms need 1 extra each.And how will they get that? So oxygen is going to share an electron with each of the hydrogens in exchange for hydrogen sharing its electron back. So they pair up to form two single bonds. And oxygen is left with 4 lone pair electrons and hydrogen with zero. They share a covalent bond in the molecule.
Hence, the required lewis structure is
What Was The Molecular Geometry For Scl6
The electron pair geometry and molecular geometry are both . All bond angles are 90. An example is sulfur hexachloride .
You May Like: Coolmath.coms
Recommended Reading: What Does Percent Error Mean In Chemistry