How To Write Chemical Formulas Correctly
A chemical formula is something like a recipe that contains different ingredients and makes an item. For example, Cocoa Butter, Chocolate Liquor, Sugar, Lecithin and a flavoring agent makes a delicious item called Chocolate. Similarly, a compound is made of several elements and an element is made of atoms.
So if you want to write a compound that shows its elements and their proportions, you have to write it in a formula. So its like a symbolic presentation of a compound using the letters and numbers.
Each symbol represents an element, and an element is made of atoms bound together by the chemical bond. The letters represent the element and the number represents the number of atoms of an element.
The formulas also contain the arrows, where one-way arrow denotes the reaction of an element towards the other element. If there is an arrow showing two sides, it means the reaction can happen in any of the forward or backward. The lines and symbols are used to from diagrams.
How Do I Add These Diagrams To My Question:
What I formerly used to do: If you want to save that image, I would suggest using the Prnt Scrn button on your computer to save the screen image.
Open Microsoft Paint, and paste it over there by pressing Ctrl V. The entire browser would get pasted as a picture. Select out the desired regions of the picture that includes the mechanism and copy that section by pressing Ctrl C.
Discard this Paint document and open another fresh one. Paste it here. Save it as a .png file and use it to upload images in your question on StackExchange.
I prefer this method as I can control the size of the picture I save by zooming the browser and/or zooming the editor.
Buttonwood pointed out that we can also lasso the molecules, using the lasso tool:
We can select the required part and right-click over it to show Save As:
Give it a suitable file name, and you got it saved!
That’s all there is, as far as I know and remember. Leave a comment if you have any doubts or need more clarification. Happy structure drawing!
Update: The editor has been changed a bit. The lower row of buttons has been shifted to the right like this:
Make sure you go into the Full Sketcher mode, which is essentially the same as before.
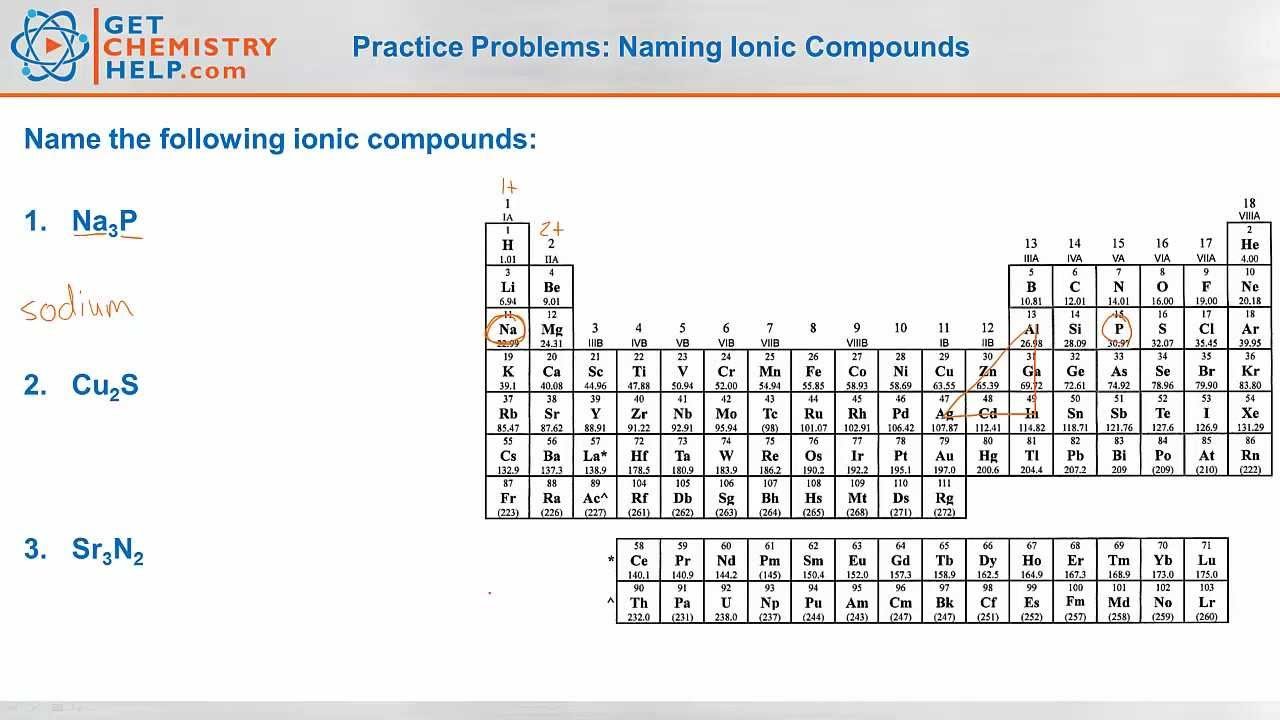
Determining The Valence Charge
You can determine the valence charge of an element by looking at its position in the element table. There are also some rules which can help you identify the valence charge if you keep in mind. They are
- All the elements of group 1 have a charge of +1
- All the elements of group 2 have a charge of +2
- The charge of transition elements is indicated by Roman numerals in parenthesis.
- The charge of silver is 1+, zinc has a 2+ and aluminum has a 3+ charge.
- All the elements of group 17 have a charge of 1-
- All the elements of group 16 have a charge of 2-
- All the elements of group 15 have a charge of 3-
Keep in mind that you should use the charge of a complete polyatomic ion instead of the individual ions when you are working with a compound with polyatomic ions.
Read also: How to ask experts to do my research paper for me and get perfect results?
Don’t Miss: Asvab Arithmetic Reasoning Worksheets
Write The Second Element
Like the first element, that can be called a first name of the compound, the second element can be called the last name. The element name will end with the suffix ide for the covalent compounds. For example Dinitrogen hexafluoride. In this compound, the second element is Fluorine and the chemical symbol of this element is F.
Same as the first element, you can identify the number of an atom from the second element name if you memorize the Greek prefixes. For example, if you are writing a formula for Dinitrogen hexafluoride, you can write the number of atoms for Fluorine by checking its Greek prefix.
The second element in this compound is hexafluoride, and the prefix is hexa. In Greek, hexa means 6, so this compound contains 6 atoms of fluorine. To write this element in a symbolic way, you can write it as F6.
This way, by writing the first and the second element using their symbols and number of their atoms, the complete chemical formula of Dinitrogen hexafluoride is N2F6.
This may seem complex and difficult initially. You may also have issues with memorizing the Greek prefixes. But as you practice more writing the formulas, you will become familiar with the element symbols and prefixes and how to write them. Here are some more examples of chemical equations. Check them to improve your understanding of the language of chemistry.
Compound: CaO Calcium oxide
Compound: SO2 Sulfur dioxide
Compound: P2O5 Diphosphorus pentoxide
What Is An Atomic Mass
Dalton in his Atomic theory further theorized that each element consisted of an atomic mass. This theory was widely accepted as it explained the law of constant proportions and the scientists were encouraged to weigh the atomic mass of the elements. However, it was soon realized that determining the mass of an atom was not an easy exercise.
Therefore, scientists developed a way of determining atomic weight by forming compounds using the law of chemical combinations. In 1961 a standard unit of Carbon atom was determined to be 12 isotopes . It was proposed that one atomic mass unit was 1/12th of the mass of the mass of a single carbon atom. Based on this relative masses of other elements were also determined.
For example, oxygen has an atomic mass of 16. Sodium has an atomic mass of 23 and accordingly other elements also received their atomic masses.
Learn more about Atomic Number here.
Read Also: How To Calculate Ihd Organic Chemistry
Balancing The Positive And Negative Charges Of The Ions
As you know now that the number of atoms of each element present in a compound is determined by the charge of each element or polyatomic ion. In order to balance the charges, you have equal them for both the elements of a compound by adding the atoms.
Take Lithium Oxide for example. This compound contains lithium and oxygen. But lithium is from group 1 and hence have a +1 charge while oxygen is from group 16 and have a 2- charge. This has to be balanced, so you will need 2 atoms of lithium. This will balance 2- charge of oxygen and the formula of this compound is Li2O.
What Are The Basics Of Chemical Reactions
- A chemical reaction is a process in which one or more substances, also called reactants, are converted to one or more different substances, known as products. Substances are either chemical elements or compounds.
- A chemical reaction rearranges the constituent atoms of the reactants to create different substances as products. The properties of the products are different from those of the reactants.
- Chemical reactions differ from physical changes, which include changes of state, such as ice melting to water and water evaporating to vapor. If a physical change occurs, the physical properties of a substance will change, but its chemical identity will remain the same.
Don’t Miss: How To Study For Ap Human Geography
Finding The Molar Mass Of A Compound
The molar mass of a compound is simply the combined molar mass of all the elements that the compound is made of. The simplest way to do this is to find the type and amount of each element in the molecule, find the molar mass of each element and then add it all together.
You can find the molar mass of each element from the periodic table. K has a molar mass of 39.1 grams/mole, O has a molar mass of 16.0 grams/mole and H has a molar mass of 1.01 grams/mole.
The molar mass of KOH is therefore 39.1 + 16.0 + 1.01 = 56.1 grams/mole.
Infographic on finding the molar mass of a compound. Released under CC-BY-SA 4.0
Writing Formulas For Ionic Compounds Containing Polyatomic Ions
Writing a formula for ionic compounds containing polyatomic ions also involves the same steps as for a binary ionic compound. Write the symbol and charge of the cation followed by the symbol and charge of the anion.
Example \: Calcium Nitrate
Write the formula for calcium nitrate.
Solution
| Crisscross Method | Write the formula for calcium nitrate |
|---|---|
| 1. Write the symbol and charge of the cation first and the anion second. | \ |
| 2. Transpose only the number of the positive charge to become the subscript of the anion and the number only of the negative charge to become the subscript of the cation. | |
| 3. Reduce to the lowest ratio. | |
| 4. Write the final formula. Leave out all subscripts that are 1. If there is only 1 of the polyatomic ion, leave off parentheses. | \ |
Example \
Write the chemical formula for an ionic compound composed of the potassium ion and the sulfate ion.
Solution
| Explanation | Answer |
|---|---|
| Potassium ions have a charge of 1+, while sulfate ions have a charge of 2â. We will need two potassium ions to balance the charge on the sulfate ion, so the proper chemical formula is K2SO4. | \ |
Write the chemical formula for an ionic compound composed of each pair of ions.
- Answer a:
- Al3
Don’t Miss: Theory Of Everything Geometry Dash 2
Why Is Nomenclature Important What Is The Purpose Of Nomenclature
There are two objectives of using nomenclature in chemistry:
- To make sure that a spoken or written chemical name does not contain any ambiguity regarding the chemical compound the name is referring towards. It is important that each chemical name points towards a single substance.
- To ascertain that each substance has one name only
- To help the chemists communicate with their peers easily.
Emma
What Is A Compound
When two or more elements chemically combine in a fixed ratio by mass, the obtained product is known as a compound. Compounds can be defined as substances consisting of 2 or more different types of elements in a fixed ratio of its atoms. When the elements combine, some individual property of the elements is lost and the newly formed compound has new properties.
Chemical Formula: Compounds are represented by their chemical formula. A chemical formula is a symbolic representation of the proportions of atoms that constitute a particular chemical compound.
The chemical formula of water is H2O which shows two atoms of hydrogen and one atom of oxygen have combined to form one molecule of H2O. The chemical formula for common salt is NaCl which shows one atom of sodium and one atom of chlorine combine to form one molecule of NaCl.
You May Like: What Causes Parallax Error And How Do You Avoid It
Formulas Of Ionic Compounds
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
Ionic compounds form when positive and negative ions share electrons and form an ionic bond. The strong attraction between positive and negative ions often produce crystalline solids that have high melting points. Ionic bonds form instead of covalent bonds when there is a large difference in electronegativity between the ions. The positive ion, called a cation, is listed first in an ionic compound formula, followed by the negative ion, called an anion. A balanced formula has a neutral electrical charge or net charge of zero.
How Do You Know Whether To Use ‘ide’ Or ‘ate’ When Naming A Compound
-ide is used for non-metal compounds generally. For example, Chlorine forms a chloride ion, so NaCl is Sodium Chloride. -ate and -ite are commonly used for polyatomic ions of Oxygen. -ate is used for the ion that has the largest number of Oxygen atoms. The -ite would be used for the ion with the smaller. NO2 and NO3 are known as Nitrite and Nitrate respectively. Nitrite has a smaller number of oxygen atoms so when added to an element it will be _ Nitrite. On the other than, Nitrate has a larger number of Oxygen atoms so when added to an element it is _ Nitrate Share your tips and advice for learning the names of chemical compounds in the comments.
Recommended Reading: What Is An Inertial Frame Of Reference In Physics
How To Write Formulas For Product Given Reactants
There will be 2 cations and anions in a double replacement equation. For example AB + CD AD + CB. Here cations are A and C and anions are B and D. Consider a real formula for an example, i.e. AgNO3 + NaCl, here Ag and Na are cations and NO3 and CI are anions.
Now the switch of the ions in the above formula, so it will look like AgCI + NaNO3. In this switch, the first cation and second anion are making a pair, and second cation and first anion are making a pair.
For example: AgNO3 + NaCl AgCI + NaNO3.
You should also mention the state of matter for both reactants and products in parenthesis. In a formula, indicates solid, indicates liquid and indicates gas. You should mention the state after each element as shown in the example below.
SnO2 + 2 H2 Sn + 2 H2O
Now you should be familiar enough to write chemical formulas. However, you can refer to more examples online and practice by writing more formulas yourself.
Receive paper in 3 Hours!
- Choose the number of pages.
- Select your deadline.
Erin Brokovich And Chromium Contamination
In the early 1990s, legal file clerk Erin Brockovich discovered a high rate of serious illnesses in the small town of Hinckley, California. Her investigation eventually linked the illnesses to groundwater contaminated by Cr used by Pacific Gas & Electric to fight corrosion in a nearby natural gas pipeline. As dramatized in the film Erin Brokovich , Erin and lawyer Edward Masry sued PG& E for contaminating the water near Hinckley in 1993. The settlement they won in 1996$333 millionwas the largest amount ever awarded for a direct-action lawsuit in the US at that time.
Figure 1. Erin Brockovich found that Cr, used by PG& E, had contaminated the Hinckley, California, water supply. The Cr ion is often present in water as the polyatomic ions chromate, CrO42 , and dichromate, Cr2O72 .
Chromium compounds are widely used in industry, such as for chrome plating, in dye-making, as preservatives, and to prevent corrosion in cooling tower water, as occurred near Hinckley. In the environment, chromium exists primarily in either the Cr or Cr forms. Cr, an ingredient of many vitamin and nutritional supplements, forms compounds that are not very soluble in water, and it has low toxicity. But Cr is much more toxic and forms compounds that are reasonably soluble in water. Exposure to small amounts of Cr can lead to damage of the respiratory, gastrointestinal, and immune systems, as well as the kidneys, liver, blood, and skin.
Recommended Reading: What Is The Formula Of Volume In Physics
Naming Ions And Ionic Compounds
Ionic compounds consist of cations and anions .The nomenclature, or naming, of ionic compounds is based on the names of the component ions. Here are the principal naming conventions for ionic compounds, along with examples to show how they are used:
Roman NumeralsA Roman numeral in parentheses, preceded by the name of the element, is used for elements that can form more than one positive ion.
This is usually seen with transition metals.Fe2+ Iron Cu2+ Copper
-ous and -icAlthough Roman numerals are used to denote the ionic charge of cations, it is still common to see and use the endings -ous or -ic. These endings are added to the Latin name of the element to represent the ions with lesser or greater charge, respectively.The Roman numeral naming convention has wider appeal because many ions have more than two valences.Fe2+ Ferrous
The -ide ending is added to the name of a monoatomic anion of an element.H- Hydride
P3 Phosphide
-ite and -ateSome polyatomic anions contain oxygen. These anions are called oxyanions. When an element forms two oxyanions, the one with less oxygen is given a name ending in -ite and the one with more oxygen is given a name that ends in -ate.NO2- NitriteSO4- Sulphate
hypo- and per-In the case where there is a series of four oxyanions, the hypo- and per- prefixes are used in conjunction with the -ite and -ate suffixes. The hypo- and per- prefixes indicate less oxygen and more oxygen, respectively.ClO- HypochloriteClO4- Perchlorate
Naming Ionic Compounds Using
Although Roman numerals are used to denote the ionic charge of cations, it is still common to see and use the endings -ous or -ic. These endings are added to the Latin name of the element to represent the ions with lesser or greater charge, respectively. The Roman numeral naming convention has wider appeal because many ions have more than two valences.
- Fe2+ Ferrous
Example: FeCl3 is ferric chloride or iron chloride.
Recommended Reading: Abiotic Science Definition
Elements Compounds And Formulae
An overview of the definitions of different types of chemicals, how chemicals can be represented in chemical equations, how chemicals can be separated, and a number of important calculations related to chemical formulae.
- A pure substance that is listed in the periodic table and only has one type of atom in it.
- There are over 100 elements.
- Most are metals, a few are metalloids , and the rest are non-metals.
What Are The General Rules For Nomenclature What Are Nomenclature Rules
Scientists employ nomenclature to name compounds clearly in chemistry. Ionic and molecular compounds are named using distinct methods. Nomenclature in chemistry refers to a set of rules to generate systematic names of compounds. The nomenclature which is used by the chemists and scientists worldwide is created and developed by the IUPAC .
Don’t Miss: Eoc Fsa Warm Ups Algebra 1 Answers