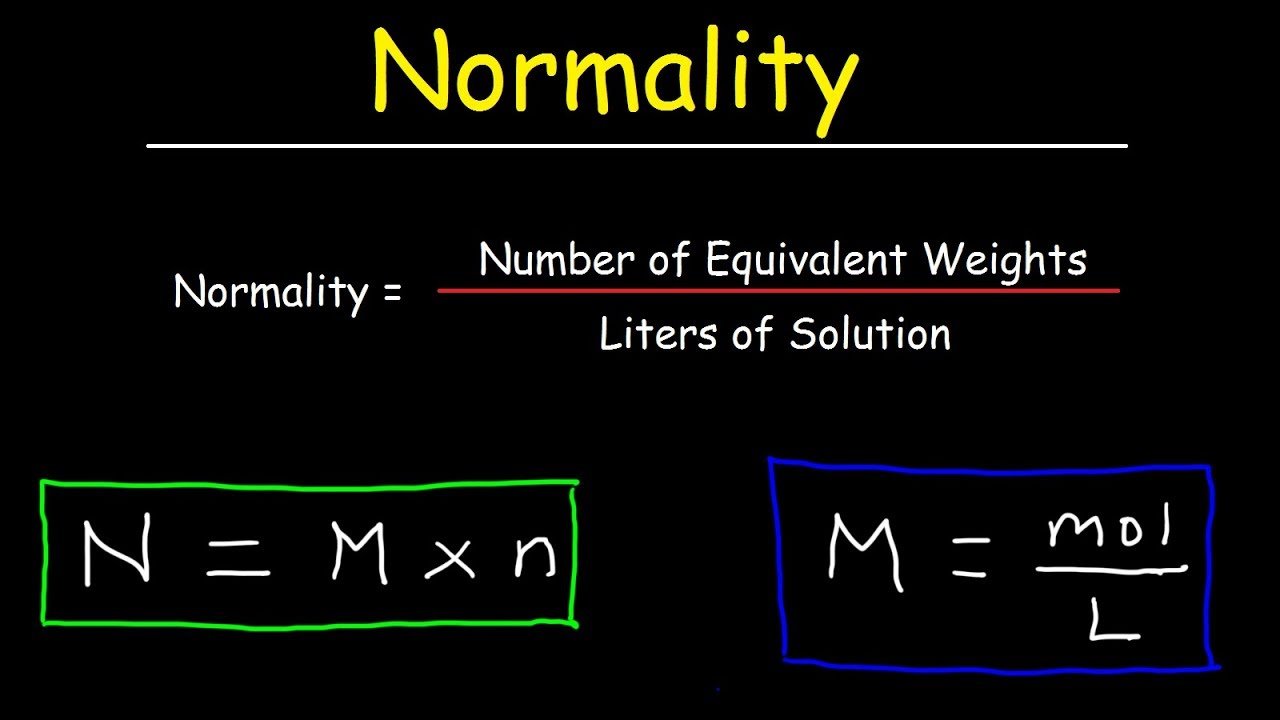
How To Calculate Normality
There are certain tips that students can follow to calculate normality.
Re: Dilution Problem With Normality
sq1009 wrote:
A 1N solution of NaOH is diluted 4/20, then rediluted 1:50. What is the final normality? How many grams of NaOH are present in 100 ml of the final solution?
I don’t know how to do normality problems
maybe this could help, read here,
It helped me solve this problem but what about say CaCl2 . In this case will the N=M since there is no H or OH ion?
Submitted by shengoc on Sun, 05/23/2010 – 15:02
When To Use Normality
There are specific circumstances when it’s preferable to use normality rather than molarity or other unit of concentration of a chemical solution.
- Normality is used in acid-base chemistry to describe the concentration of hydronium and hydroxide . In this situation, 1/feq is an integer.
- The equivalence factor or normality is used in precipitation reactions to indicate the number of ions that will precipitate. Here, 1/feq is once again and integer value.
- In redox reactions, the equivalence factor indicates how many electrons can be donated or accepted by an oxidizing or reducing agent. For redox reactions, 1/feq may be a fraction.
Don’t Miss: Exponential Growth And Decay Common Core Algebra 1 Homework Answers
Normality Problems And Examples
Question 1. In the following reaction calculate and find the normality when it is 1.0 M H3PO4
H3AsO4 + 2NaOH Na2HAsO4 + 2H2O
Solution:
If we look at the given reaction we can identify that only two of the H+ ions of H3AsO4 react with NaOH to form the product. Therefore, the two ions are 2 equivalents. In order to find the normality, we will apply the given formula.
N = Molarity × number of equivalents
N = 1.0 × 2
Therefore, normality of the solution = 2.0.
Question 2. Calculate the normality of the solution obtained by dissolving 0.321 g of the salt sodium carbonate in 250 mL water.
Solution:
Question 3. What is the normality of the following?
- 0.1381 M NaOH
a. N = 0.1381 mol/L × = 0.1381 eq/L = 0.1381 N
b. N = 0.0521 mol/L × = 0.156 eq/L = 0.156 N
Question 4. What will the concentration of citric acid be if 25.00 ml of the citric acid solution is titrated with 28.12 mL of 0.1718 N KOH?
Solution:
Na × =
Therefore, the concentration of citric acid = 0.1932 N.
Question 5. Find the normality of the base if 31.87 mL of the base is used in the standardization of 0.4258 g of KHP ?
Solution:
0.4258 g KHP × × :
= 2.085 × 10-3 eq base/0.03187 L = 0.6542 N
Normality of the base is = 0.6542 N.
Question 6. Calculate the normality of acid if 21.18 mL is used to titrate 0.1369 g Na2CO3?
Solution:
0.1369 g Na2CO3 × × × :
= 2.583 × 10-3 eq acid/0.02118 L = 0.1212 N
Normality of the acid = 0.1212 N.
Try this:
Answer: 6.0 M Al3+.
Normality Calculator For Concentrated Liquid Chemical

This is very handy tool for science student to make reagents for analysis. You can easily calculate normality of any concentrated acid or base liquid solution by using this normality calculator.
In conclusion, Normality or equivalent concentration of the solution indicates how many equivalent are present per liter of solution.
Recommended Reading: What Is The Molecular Geometry Of Ccl4
How Is Concentration Measured In Chemistry
First, it is necessary to understand how atoms and molecules combine in chemical reactions, which is not quite like the “ingredients” in, say a cooking recipe or a construction project. Ordinarily, you would keep track of different objects by measuring their masses or perhaps their volumes.
So, if you know that 1 kg of flour and 0.5 kg of sugar is enough to make 10 servings of a particular dessert product, you know that 100 kg of flour and 50 kg of sugar are enough for 1,000 servings. And in mixing drinks, if 50 mL of your own homemade fitness water needs 5 mL of vinegar, then 1,000 mL of the product needs 20 times this amount of of the vinegar reagent is 100 mL.
With reactions, it’s whole particles you need to count. These are measured in moles, and translating them into this “language” from mass only requires you to know the molar masses of the constituent atoms.
S Per Million And Parts Per Billion
Parts per million and parts per billion are ratios that give the grams of solute in, respectively, one million or one billion grams of sample. For example, a sample of steel that is 450 ppm in Mn contains 450 g of Mn for every gram of steel. If we approximate the density of an aqueous solution as 1.00 g/mL, then we can express solution concentrations in ppm or ppb using the following relationships.
For gases a part per million usually is expressed as a volume ratio for example, a helium concentration of 6.3 ppm means that one liter of air contains 6.3 L of He.
You should be careful when using parts per million and parts per billion to express the concentration of an aqueous solute. The difference between a solutes concentration in mg/L and ng/g, for example, is significant if the solutions density is not 1.00 g/mL. For this reason many organizations advise against using the abbreviation ppm and ppb . If in doubt, include the exact units, such as 0.53 g Pb2+/L for the concentration of lead in a sample of seawater.
Don’t Miss: Fsa Algebra 1 Eoc Review Functions And Modeling-answer Key
What Is A Mole
A mole of anything, such as atoms, molecules, marbles or giraffes, is equal to 6.022 × 1023 individual instances of that thing. This happens to be the number of particles in exactly 12 grams of the most common form of the element carbon, number 6 on the periodic table of elements. The number of grams in 1 mole of a given element is under its symbol in its personalized “box” on the table.
To get the molar mass of a molecule, or the mass of 1 mol of those molecules, simply add the individual masses of the atoms, being sure to account for subscripts. So for a water molecule, H2O, you would add the mass of 1 mol O to the mass of 2 mol H to get about 18.015 g.
Empirical And Molecular Formulas
- Chemicalaid Empirical Formulas – Perfect when you need to know the empirical formula of an equation and need the molecular formula, or vice versa.
- Easycalculation Chemical Formulas – Click here for simple and efficient calculations for the percentage of each element in a given compound.
- Empirical and Molecular Formula Solver – Want to make sure all your empirical and molecular ducks are in a row? Simply follow the easy instructions on mmsphyschem.com and you’ll master this aspect of chemistry in no time.
- University of Sydney Empirical Formula Calculator – Enter your empirical formulas here and get back the percentage of mass for each element involved.
Read Also: Lesson 1.7 Practice A Geometry Answers
How To Calculate Normality Of Concentrated Hydrochloric Acid
First, note down three things form packing label of solution:
Use below formula to calculate normality of any commercially available liquid solution:
Normality = Specific gravity × Percentage of purity in decimal × 1000 ÷ Equivalent weight
If you see label of reagent bottle of hydrochloric acid you will get below three values:
Put the above values in formula,
Normality = 1.18 × 0.354 × 1000 ÷ 36.46 = 11.46 N
How To Calculate Normality From Molarity
The mole equivalents of an acid or base are calculated by determining the number of H+ or OH- ions per molecule: N = n × M
For an acid solution, n is the number of H+ ions provided by a formula unit of acid.Example: A 3 M H2SO4 solution is the same as a 6 N H2SO4 solution.
For a basic solution, n is the number of OH- ions provided by a formula unit of base.Example: A 1 M Ca2 solution is the same as a 2 N Ca2 solution.
Note:The normality of a solution is NEVER less than its molarity!
Also Check: Illest Road Trip Of All Time
How To Calculate Normality Of A Solution
Normality is a unit of the concentration of a chemical solution defined as the gram equivalent weight of solute per liter of solution. Normality is also called equivalent concentration. It is indicated by the symbol N or eq/L . To find the gram equivalent weight, you need to know how many hydrogen ions , hydroxide ions , or electrons are transferred in a reaction or you need to know the valence of the chemical species.
The International Union of Pure and Applied Chemistry discourages the use of this unit, but you may encounter it in chemistry classes or the lab, particularly with acid-base titrations and redox reactions. Here is a look at the different ways to calculate normality of solution, along with examples.
What Is Valency In Chemistry
The valency or valence of an atom or molecule described as how may hydrogen atoms it can bond with. In our example,
Hydrochloric Acid HCl have a one hydrogen atom so, the equivalents of Hydrochloric Acid is 36.46/1 = 36.46
Sulphuric Acid H2SO4 have two hydrogen atoms so, the equivalents of Sulphuric Acid is 98/2 = 49
Recommended Reading: Unit 1 Test Geometry Basics Answers Key
Is There Any Way To Solve Normality Problem In R
I am trying to do multiple regression test in R.I have made a model but I have found some problems.I have done homogeneity of variance test and the variance was not found to be heterogeneous.But I have done normality test and p-value was very low. It was about 2e-16.Here’s the data and my script.
Should I convert my data? Should I use boxcox?Any help should be appreciated.
S For Solving Normality Problems
Also Check: What Is The Molecular Geometry Of Ccl4
Normality Definition And Formula
MolarityMole FractionMolalityEquivalent Weight Definition and Gram Equivalent WeightMolar MassFew more important things about Normaility
- Normality unit is N or eq/L .It depends on the chemical reaction being studied and depends on temperature too. So ,it is meaning with respect to the chemical reaction
- It is the reactive capacity of the solution and it is called equivalent concentration
- This is used in acid-base reactions and redox reactions
Converting Between Concentration Units
The most common ways to express concentration in analytical chemistry are molarity, weight percent, volume percent, weight-to-volume percent, parts per million and parts per billion. The general definition of concentration in Equation \ref makes it is easy to convert between concentration units.
Example 2.2.1
A concentrated solution of ammonia is 28.0% w/w NH3 and has a density of 0.899 g/mL. What is the molar concentration of NH3 in this solution?
Solution
Example 2.2.2
The maximum permissible concentration of chloride ion in a municipal drinking water supply is \ ppm Cl. When the supply of water exceeds this limit it often has a distinctive salty taste. What is the equivalent molar concentration of Cl?
Solution
Exercise 2.2.1
Which solution0.50 M NaCl or 0.25 M SrCl2has the larger concentration when expressed in mg/mL?
- Answer
-
The concentrations of the two solutions are
The solution of SrCl2 has the larger concentration when it is expressed in g/mL instead of in mol/L.
Recommended Reading: Unit 1 Geometry Basics Homework 2
How To Calculate Normality In Chemistry
When you hear the word “normality,” science may not be the first thing that comes to mind. “Normal,” to most people, means “ordinary” or “typical,” and doesn’t seem to have a whole lot of scientific weight. But in chemistry, the term normality is closely related to a few core chemistry concepts, and in particular, it is vital in the area of solutions chemistry.
If you see “normal” as “expected,” even in the chemical sense this is more or less on target: A normalized preparation is one that has been created in proportion or relation to an established standard. To discover how to calculate the normality of NaOH, or how to convert from normality to molarity , read on!
Relationship Between Normality And Molarity
I found this formula for calculating the normality from the molarity:$$\text = n \cdot \text$$
with$$n = \frac}}$$
But in one book I found that if equivalent weight is equal to half of the molecular weight then$$2N = M$$
Under the given condition, I am confused if the above statement is true.
Am I following a factually correct book for solving problems on volumetric analysis?
The normality of a solution is the molarity of the solution multiplied by the number of equivalents per mole:
$$\mathrm = \mathrm}\cdot\mathrm= \mathrm}\cdot\mathrm}=\mathrm}$$
Example: Calculate the normality of a 1 $\mathrm$ solution of $\ce$. There are 2 equivalents per mole, from the reaction
$$\ce}$$
which yields 2 moles of $\ce$ for every 1 mole of $\ce$.
Using the relation given above, we find that a 1 $\mathrm$ solution of $\ce$ is 2 $\mathrm$:
$$\mathrm}\cdot\mathrm=2\ \mathrm$$
One resource you might find useful in order to check your math is Sigma-Aldrich’s Normality & Molarity Calculator. Additionally, there are details relevant to the definitions of molarity and normality that you might find clearer that the ones you’ve conveyed in your question.
Also Check: What Is The Molecular Geometry Of Ccl4
Setting Your Browser To Accept Cookies
There are many reasons why a cookie could not be set correctly. Below are the most common reasons:
- You have cookies disabled in your browser. You need to reset your browser to accept cookies or to ask you if you want to accept cookies.
- Your browser asks you whether you want to accept cookies and you declined. To accept cookies from this site, use the Back button and accept the cookie.
- Your browser does not support cookies. Try a different browser if you suspect this.
- The date on your computer is in the past. If your computer’s clock shows a date before 1 Jan 1970, the browser will automatically forget the cookie. To fix this, set the correct time and date on your computer.
- You have installed an application that monitors or blocks cookies from being set. You must disable the application while logging in or check with your system administrator.
Limitations In Using Normality
Many chemists use normality in acid-base chemistry to avoid the mole ratios in the calculations or simply to get more accurate results. While normality is used commonly in precipitation and redox reactions there are some limitations to it. These limitations are as follows:
- It is not a proper unit of concentration in situations apart from the ones that are mentioned above. It is an ambiguous measure and molarity or molality are better options for units.
- Normality requires a defined equivalence factor.
- It is not a specified value for a particular chemical solution. The value can significantly change depending on the chemical reaction. To elucidate further, one solution can actually contain different normalities for different reactions.
Read Also: Segment Addition Postulate Practice Answer Key
Number Of Gram Equivalent
So, lets get forward with understanding the concept of gram equivalence.
We know that no of moles =\
Number of gram equivalent = \, and equivalent weight = \
Well understand these two formulas with an example.
Lets find out Gram Equivalent
For example, Find the number of gram equivalents present in 0.5 g of HCl.
HCl releases one H+ ion in the solution, so its valence factor = 1.
The molecular weight of HCl = 36.46 g.
So, equivalent weight = \ = 36.46/1 = 36.46 g, and
Number of gram equivalent = \ = 0.5/36.46 = 0.0137
So, we get the number of gram equivalent = 0.0137
Let us take another example of 1.06 g of Na2CO3 to understand this concept clearly
We are given the mass of Na2CO3 = 1.06 g.
Firstly, Find the equivalent weight of Na2CO3.
Since Na2CO3 is a salt, so the number of positive charges on the cation gives X = 2
Molecular weight = 106 g
So, Equivalent Weight = \ = 106/2 = 53 g, and number of gram equivalent is:
= \ = 1.06/53 = 0.02
In a chemical equation, the number of gram equivalent of both reactions always remains the same.
What Gets Stored In A Cookie
This site stores nothing other than an automatically generated session ID in the cookie no other information is captured.
In general, only the information that you provide, or the choices you make while visiting a web site, can be stored in a cookie. For example, the site cannot determine your email name unless you choose to type it. Allowing a website to create a cookie does not give that or any other site access to the rest of your computer, and only the site that created the cookie can read it.
Read Also: Who Are Paris Jackson’s Biological Parents
Differences Between Normality And Molarity
Here are some key differences between normality and molarity.
| Normality | |
| It is defined as the number of gram equivalent per litre of solution. | It is defined as the number of moles per litre of solution. |
| It is used in measuring the gram equivalent in relation to the total volume of the solution. | It is used in measuring the ratio between the number of moles in the total volume of the solution. |
| The units of normality are N or eq L-1 | The unit of molarity is M or Moles L-1 |