How To Calculate The Spring Constant
There are two simple approaches you can use to calculate the spring constant, using either Hookes law, alongside some data about the strength of the restoring force and the displacement of the spring from its equilibrium position, or using the elastic potential energy equation alongside figures for the work done in extending the spring and the displacement of the spring.
Using Hookes law is the simplest approach to finding the value of the spring constant, and you can even obtain the data yourself through a simple setup where you hang a known mass from a spring and record the extension of the spring. Ignoring the minus sign in Hookes law and dividing by the displacement, x, gives:
Using the elastic potential energy formula is a similarly straightforward process, but it doesnt lend itself as well to a simple experiment. However, if you know the elastic potential energy and the displacement, you can calculate it using:
In any case youll end up with a value with units of N/m.
How To Use Coulomb’s Law
Coulomb’s law, otherwise known as Coulomb’s inverse-square law, describes the electrostatic force acting between two charges. The force acts along the shortest line that joins the charges. It is repulsive if both charges have the same sign and attractive if they have opposite signs.
Coulomb’s law is formulated as follows:
where:
- F is the electrostatic force between charges ,
- q is the magnitude of the first charge ,
- q is the magnitude of the second charge ,
- r is the shortest distance between the charges ,
- ke is the Coulomb’s constant. It is equal to 8.98755 × 10 N·m²/C². This value is already embedded in the calculator – you don’t have to remember it 🙂
Simply input any three values into our electric force calculator to obtain the fourth as a result.
To compute the electric potential at a point either due to a single point charge or a system of point charges, check out our electric potential calculator.
Boltzmann Constant In Ev
The Boltzmann constant is calculated by dividing the gas steady R by Avogadro’s number NA. Boltzmann constant k or kB = 1.3806452 x 1023 J/K is the estimate of k or kB.
In eV, the Boltzmann constant is 8.6173303 x 10-5 eV/K.
The Boltzmann constant can be represented in a variety of different units. The value of k, as well as other units, are listed in the table below.
| Value Of k | |
|---|---|
| 1.0 | Atomic unit |
The Boltzmann constant serves as a link between the microscopic energy E and the macroscopic temperature scale.
T = E/k
Setting k to unity, which results in temperature and energy numbers of the same kind, is a common practice in physics. Temperature is essentially measured in units of energy in this situation.
Read More: Continuous Charge Distribution
Read Also: Klohe Kardashians Real Father
What Is The Friction Coefficient
The Friction coefficient is a constant for a pair of surfaces in contact which decides the amount of frictional force generated at the common layer when one surface moves or tends to move with respect to the other surface. This coefficient is presented generally in this way: = F /N= ratio of frictional force and Normal reaction force. It has 2 different sets of values for a pair of surfaces and these are known as 1] the Coefficient of static frictions and 2] the Coefficient of Kinetic frictionk
Fundamental Constants And The Si System Of Units:
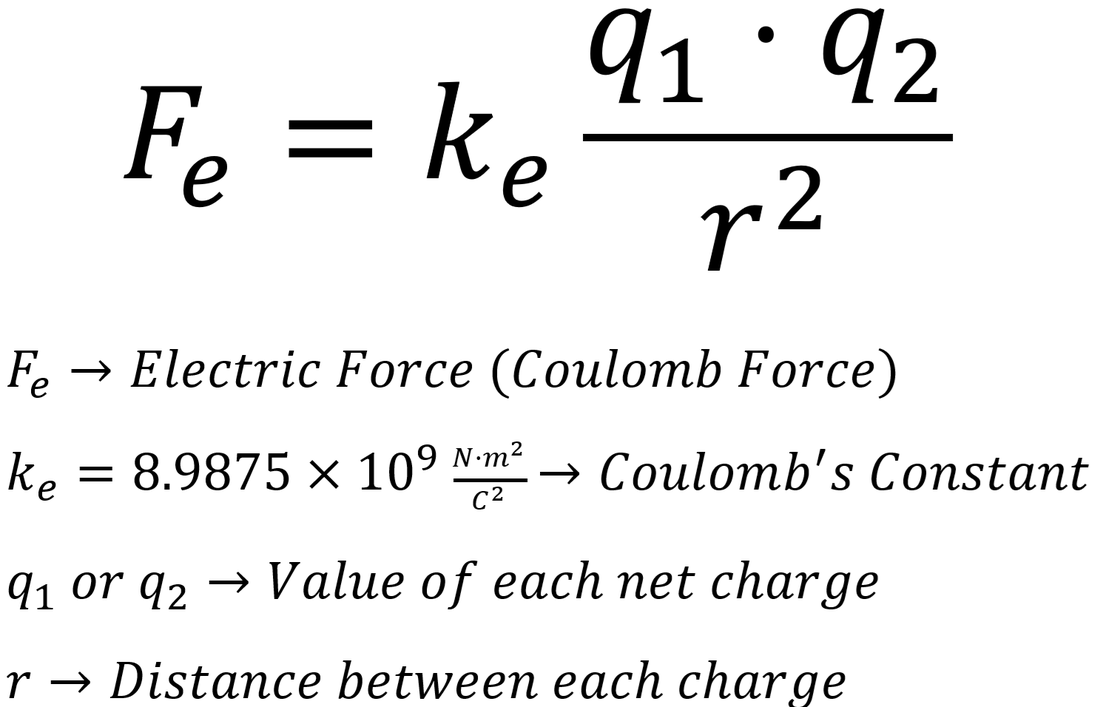
Each fundamental constant Q is a product of a number and a base unit :
Q = x ,
for example Boltzmanns constant is:k = 1.380 649 x 10-23 JK-1.
Thus we have two ways to define the SI system of SI base units:
Read Also: Segment And Angle Addition Worksheet Answers
Elastic Potential Energy Equation
The concept of elastic potential energy, introduced alongside the spring constant earlier in the article, is very useful if you want to learn to calculate k using other data. The equation for elastic potential energy relates the displacement, x, and the spring constant, k, to the elastic potential PEel, and it takes the same basic form as the equation for kinetic energy:
As a form of energy, the units of elastic potential energy are joules .
The elastic potential energy is equal to the work done , and you can easily calculate it based on the distance the spring has been stretched if you know the spring constant for the spring. Similarly, you can re-arrange this equation to find the spring constant if you know the work done in stretching the spring and how much the spring was extended.
The Friction Coefficient Table Sample Values For Different Materials
| System |
values
As an example, if we take the value of s and k for 2 wood surfaces in contact, we can easily observe that k is less than s. This is true for all other material pairs in the above list. This clearly explains why the kinetic friction magnitude is less than static friction.Also find 2 interesting readings above: Steel on dry steel and Steel on oiled steel. It shows how lubrication reduces the value of friction.
Don’t Miss: Kuta Software Infinite Algebra 2 Rationalizing Imaginary Denominators
Quick Answer: What Is K In Physics
Boltzmann constant, , a fundamental constant of physics occurring in nearly every statistical formulation of both classical and quantum physics. The molar gas constant R is defined as Avogadros number times the Boltzmann constant.
What is k constant unit?
- The spring constant, k, in Hookes Law has units of Newton per meter since it is a constant for the force applied per unit of length.
Applications Of Boltzmann Constant
There is no way to determine the state of each individual molecule in a large ensemble of objects, such as the billions of trillions of hot molecules propelling a piston in a steam engine as they are moving at different velocities with a range of different energies. Boltzmann Constant is used in several branches of material science. Some of them are as listed below:
- In classical factual mechanics, the Boltzmann Constant is used to express the proportionality of a molecule’s energy.
- Boltzmann factors are communicated using this constant.
- It plays an important role in the definition of entropy.
- Boltzmann Constant is used for transmitting warm voltage in semiconductor material research.
Read More:Physical Significance of Electric Field
Read Also: Paris Jackson Real Parents
What Is K In Physics Spring
k is the spring constant, in Newtons per meter , and x is the displacement of the spring from its equilibrium position. The spring constant, k, is representative of how stiff the spring is. The displacement of an object is a distance measurement that describes that change from the normal, or equilibrium, position.
What Is The Value Of K For Air
Neha Gupta answered this
Dezinerr answered this
The nominal values used for air at 300 K are CP= 1.00 kJ/kg.K, Cv= 0.718 kJ/kg.K,, and k = 1.4. However they are all functions of temperature, and with the extremely high temperature range experienced in internal combustion and gas turbine engines one can obtain significant errors. The table following gives the values of specific heat capacities as a function of temperature. We find that choosing values of specific heat capacities at the average temperature of each process gives results with reasonable accuracy .
Recommended Reading: New England Climate And Geography
The Spring Constant: Car Suspension Problem
A 1800-kg car has a suspension system that cannot be allowed to exceed 0.1 m of compression. What spring constant does the suspension need to have?
This problem might appear different to the previous examples, but ultimately the process of calculating the spring constant, k, is exactly the same. The only additional step is translating the mass of the car into a weight on each wheel. You know that the force due to the weight of the car is given by F = mg, where g = 9.81 m/s2, the acceleration due to gravity on Earth, so you can adjust the Hookes law formula as follows:
However, only one quarter of the total mass of the car is resting on any wheel, so the mass per spring is 1800 kg / 4 = 450 kg.
Now you simply have to input the known values and solve to find the strength of the springs needed, noting that the maximum compression, 0.1 m is the value for x youll need to use:
This could also be expressed as 44.145 kN/m, where kN means kilonewton or thousands of newtons.
Role In The Equipartition Of Energy
Given a thermodynamic system at an absolute temperatureT, the average thermal energy carried by each microscopic degree of freedom in the system is 1/2kT .
In classicalstatistical mechanics, this average is predicted to hold exactly for homogeneous ideal gases. Monatomic ideal gases possess three degrees of freedom per atom, corresponding to the three spatial directions. According to the equipartition of energy this means that there is a thermal energy of 3/2kT per atom. This corresponds very well with experimental data. The thermal energy can be used to calculate the root-mean-square speed of the atoms, which turns out to be inversely proportional to the square root of the atomic mass. The root mean square speeds found at room temperature accurately reflect this, ranging from 1370 m/s for helium, down to 240 m/s for xenon.
Kinetic theory gives the average pressure p for an ideal gas as
- p }}m}}.}
Combination with the ideal gas law
- p
shows that the average translational kinetic energy is
- 1 . }m}}=}kT.}
Considering that the translational motion velocity vector v has three degrees of freedom gives the average energy per degree of freedom equal to one third of that, i.e. 1/2kT.
Recommended Reading: How Did The Geography Affect The Development Of Greece
What Is The Maximum Speed Of Light
But Einstein showed that the universe does, in fact, have a speed limit: the speed of light in a vacuum . Nothing can travel faster than 300,000 kilometers per second . Only massless particles, including photons, which make up light, can travel at that speed.
What is V in refractive index? In optics, the refractive index of a material is a dimensionless number that describes how fast light travels through the material. It is defined as. where c is the speed of light in vacuum and vis is the phase velocity of light in the medium.
Is time a frequency?
Frequency is a rate quantity. Period is a time quantity. Frequency is the cycles/second. Period is the seconds/cycle.
What is Alpha in circular motion? In physics, angular acceleration refers to the time rate of change of angular velocity. Angular acceleration is measured in units of angle per unit time squared , and is usually represented by the symbol alpha .
Friction: Harmful And Helpful
Friction is both harmful and helpful to you and to the world around you. Friction can cause holes in your socks.Friction by wind and water can cause erosion. On the other hand, friction between your pencil and your paper is needed for the pencil to leave a mark.
Without friction, you would slip and fall when you tried to walk. Because friction can be both harmful and helpful, sometimes it should be decreased and sometimes it should be increased.
Read Also: Molecular Geometry Report Sheet Answer Key
Value Of The Constant
The Coulomb constant is the constant of proportionality in Coulomb’s law,
- F =k_}}}\mathbf } _}
where êr is a unit vector in the r-direction. In SI:
- k }=}},}
where is the vacuum permittivity. This formula can be derived from Gauss’ law,
- S 0 \cdot }\mathbf =}}}
Taking this integral for a sphere, radius r, centered on a point charge, the electric field points radially outwards and is normal to a differential surface element on the sphere with constant magnitude for all points on the sphere.
- S r 2 \cdot }\mathbf =|\mathbf |\int _dA=|\mathbf |\times 4\pi r^}
Noting that E = F/q for some test charge q,
- F 0 \mathbf & =}}}}\mathbf } _=k_}}}\mathbf } _\\\therefore k_}& =}}\end}}
Coulomb’s law is an Inverse Square Law, and thereby similar to many other scientific laws ranging from gravitational pull to light attenuation. This law states that a specified physical quantity is inversely proportional to the square of the distance.
kkkcc
- k
How Do You Explain Coulomb’s Law
According to Coulomb, the electric force for charges at rest has the following properties: Like charges repel each other unlike charges attract. Thus, two negative charges repel one another, while a positive charge attracts a negative charge. The attraction or repulsion acts along the line between the two charges.
Read Also: What Causes Parallax Error And How Do You Avoid It
Is 53 Or 62 Engine Better
Additionally What is KBT physics? kcalmol-1. h/kT = 0.16. ps. kT is the product of the Constant Boltzmann, k , and the temperature, T.
What is P physics? Power is the rate with respect to time at which work is done it is the time derivative of work: where P is power, W is work, and t is time. If a constant force F is applied throughout a distance x, the work done is defined as .
Understanding The ‘fixing’ Of The Value Of H
Since 2019, the numerical value of the Planck constant has been fixed, with a finite decimal representation. Under the present definition of the kilogram, which states that “The kilogram is defined by taking the fixed numerical value of h to be 6.62607015×1034 when expressed in the unit Js, which is equal to kgm2s1, where the metre and the second are defined in terms of speed of lightc and duration of hyperfine transition of the ground state of an unperturbed caesium-133 atom Cs.” This implies that mass metrology is now aimed to find the value of one kilogram, and thus it is kilogram which is compensating. Every experiment aiming to measure the kilogram , will essentially refine the value of a kilogram.
As an illustration of this, suppose the decision of making h to be exact was taken in 2010, when its measured value was 6.62606957×1034 Js, thus the present definition of kilogram was also enforced. In the future, the value of one kilogram must be refined to 6.62607015/6.62606957 1.0000001 times the mass of the International Prototype of the Kilogram .
Also Check: Quantum Physics Proves God
Introducing The Spring Constant K
The size of the relationship between the extension and the restoring force of the spring is encapsulated in the value the spring constant, k. The spring constant shows how much force is needed to compress or extend a spring by a given distance. If you think about what this means in terms of units, or inspect the Hookes law formula, you can see that the spring constant has units of force over distance, so in SI units, newtons/meter.
The value of the spring constant corresponds to the properties of the specific spring under consideration. A higher spring constant means a stiffer spring thats harder to stretch , while a looser spring thats easier to stretch will have a lower spring constant. In short, the spring constant characterizes the elastic properties of the spring in question.
Elastic potential energy is another important concept relating to Hookes law, and it characterizes the energy stored in the spring when its extended or compressed that allows it to impart a restoring force when you release the end. Compressing or extending the spring transforms the energy you impart into elastic potential, and when you release it, the energy is converted into kinetic energy as the spring returns to its equilibrium position.
What Is The Boltzmann Constant
Boltzmann Constant or Boltzmanns Constant is defined as the link between absolute temperature and the kinetic energy contained in each molecule of an ideal gas, symbolized by k or kB. This constant is equal to the ratio of the gas constant to the Avogadros constant and is named after the Austrian scientist Ludwig Boltzmann .
Boltzmann Constant
The energy of a gas molecule is proportional to the absolute temperature in most cases. The kinetic energy per molecule increases as the temperature rises. When a gas is heated, the molecules in it move faster. If the gas is contained in a constant volume space, this results either in higher pressure, or an increased volume if the pressure remains constant.
The physical importance of the Boltzmann constant, k is that it gives a measure of the amount of energy associated with the random thermal movements of the particles that make up a material. The average energy per degree of freedom for a classical system in equilibrium at temperature T is kT/2. Each atom has three translational degrees of freedom in the simplest case of a gas made up of N non-interacting atoms, therefore the total thermal energy of the gas is 3NkT/2.
Read Also: Broward County Public Schools Algebra 1 Countdown Answers
What Is K In Kq1q2 R 2
the constant k = 8.99 x 109 N m2 / C2. Remember that force is a vector. When more than one charge exerts a force on another charge, the net force on that charge is the vector sum of the individual forces. The force between charges is very similar to the gravitational force between interacting masses.