Difference Between Accuracy And Precision In Chemistry
June 25, 2018 Posted by Madhu
The key difference between accuracy and precision in chemistry is that accuracy reflects how close a measurement to an accepted value whereas precision reflects how reproducible the measurements are.
Both the terms accuracy and precision gives the idea of how close a measurement is close to an actual value. But they are different from each other in definition and application. In chemistry, we use both these terms as analytical indicators for the values we get for certain experiments.
Example : Calculation With Significant Figures
One common bathtub is 13.44 dm long, 5.920 dm wide, and 2.54 dm deep. Assume that the tub is rectangular and calculate its approximate volume in liters.
\beginV\hfill & =\hfill & l\times w\times d\hfill \\ & =\hfill & \text\times \text\times \text\hfill \\ & =\hfill & \text }^\left\hfill \\ & =\hfill & }^\text\left\hfill \end
Check Your Learning
What is the density of a liquid with a mass of 31.1415 g and a volume of 30.13 cm3?
How Do You Track And Measure Accuracy And Precision
To know if you are accurate or precise, youll need to track and measure your results. Accuracy and precision are measured differently:
Accuracy measurement: How your result compares to the target value. The closer you are, the more accurate you are. To determine accuracy, you need to have clearly defined goals or success metrics youre trying to reach.
Precision measurement: How close measurements are to each other. To review for precision, youll need to develop a tracking system that shows how multiple results or data points compare to each other over time.
When it comes to a measurement system, youll want something thats easy to manage and accurate. A project management software with universal reporting can help you trackand react toresults in real-time. For example, if you notice consistent results moving away from your target goal , you may have a systematic error that needs correction.
Read Also: What Is The Definition Of Absolute Value In Math Terms
How Do You Calculate Precision And Accuracy
How to measure accuracy and precision
What Is The Difference Precision And Accuracy
Accuracy is the level of agreement between the absolute measurement and the actual measurement. Precision implies the variation level that lies in the values of several measurements of a similar factor. It represents how the results agree with the standard value closely.
What is precision in science? Related Questions
Don’t Miss: Geometry Sin Cos Tan Chart
What Is Accuracy In Chemistry With Example
Accuracy refers to the closeness of a measured value to a standard or known value. For example, if in lab you obtain a weight measurement of 3.2 kg for a given substance, but the actual or known weight is 10 kg, then your measurement is not accurate. In this case, your measurement is not close to the known value.
What Is The Difference Between Accuracy And Precision And Which Is Most Important To Scientific Measurement Quizlet
What is the difference between accuracy and precision and which is most important to scientific measurement? Accuracy describes the difference between the measurement and the parts actual value, while precision describes the variation you see when you measure the same part repeatedly with the same device.
Also Check: What Is The Meaning Of Depression In Geography
What Is The Definition Of Precision In Science
Precision refers to how close measurements of the same item are to each other. Precision is independent of accuracy. That means it is possible to be very precise but not very accurate, and it is also possible to be accurate without being precise. The best quality scientific observations are both accurate and precise.
How Can Precisa Help You With Your Precision Measurements
At Precisa, ensuring the precision of your measurements is our top priority.
Not only do we produce analytical and precision balances which measure up to 0.1 mg, but we also offer calibration services across the UK which guarantee that your readings are 100% precise and accurate.
Please browse our range of precision measurement products here and find out how our door to door UKAS accredited calibration services can give you a piece of mind.
Read Also: Khan Academy Answers Algebra 1
How Do You Calculate Accuracy Example
Accuracy: Of the 100 cases that have been tested, the test could determine 25 patients and 50 healthy cases correctly. Therefore, the accuracy of the test is equal to 75 divided by 100 or 75%. Sensitivity: From the 50 patients, the test has only diagnosed 25. Therefore, its sensitivity is 25 divided by 50 or 50%.
What Is Precision And Significant Figures
The significant figures of a number are those digits that carry meaning contributing to its precision. Thus the number of significant digits depends on the least count of the measuring instrument. All the certain digits and the one uncertain digit are called the significant figures in the measured value.
Don’t Miss: How The Mind Works Psychology
Example Of The Difference Between Accuracy And Precision
The example of a darts board is often used when talking about the difference between accuracy and precision.
Accurately hitting the target means you are close to the centre of the target, even if all the marks are on different sides of the centre. Precisely hitting a target means all the hits are closely spaced, even if they are very far from the centre of the target.
What Is Precise But Not Accurate Example
More Examples If the thermometer consistently registers the exact temperature for several days in a row, the thermometer is also precise. Precise, but not accurate: A refrigerator thermometer is read ten times and registers degrees Celsius as: 39.1, 39.4, 39.1, 39.2, 39.1, 39.2, 39.1, 39.1, 39.4, and 39.1.
Recommended Reading: Abstract Algebra Online Course For Credit
What Is The Difference Between Accuracy And Precision In Chemistry
Accuracy in chemistry refers to how close a measurement to the true value. In order to increase the accuracy, we have to calibrate the instrument that we use to take the measurement. Precision in chemistry is the reproducibility of a measurement. It gives the closeness of data in a dataset. Moreover, it is independent of accuracy.
What Is The Difference Between Accuracy And Precision Measurements
The terms Accuracy and Precision are critical within science and their meanings are constantly being used incorrectly or misunderstood to mean the same thing.
At Precisa, our work is centred on the production of Precision measurements. This article aims to clear up the confusion, exploring the difference between precision and accuracy and the correct way to use each term, using the example of a dart board to demonstrate.
Read Also: What Kind Of Jobs Can You Get With Psychology Degree
Accuracy Precision And Calibration
Do you think it’s better to use an instrument that records accurate measurements or one that records precise measurements? If you weigh yourself on a scale three times and each time the number is different, yet it’s close to your true weight, the scale is accurate. Yet it might be better to use a scale that is precise, even if it is not accurate. In this case, all the measurements would be very close to each other and “off” from the true value by about the same amount. This is a common issue with scales, which often have a “tare” button to zero them.
While scales and balances might allow you to tare or make an adjustment to make measurements both accurate and precise, many instruments require calibration. A good example is a thermometer. Thermometers often read more reliably within a certain range and give increasingly inaccurate values outside that range. To calibrate an instrument, record how far off its measurements are from known or true values. Keep a record of the calibration to ensure proper readings. Many pieces of equipment require periodic calibration to ensure accurate and precise readings.
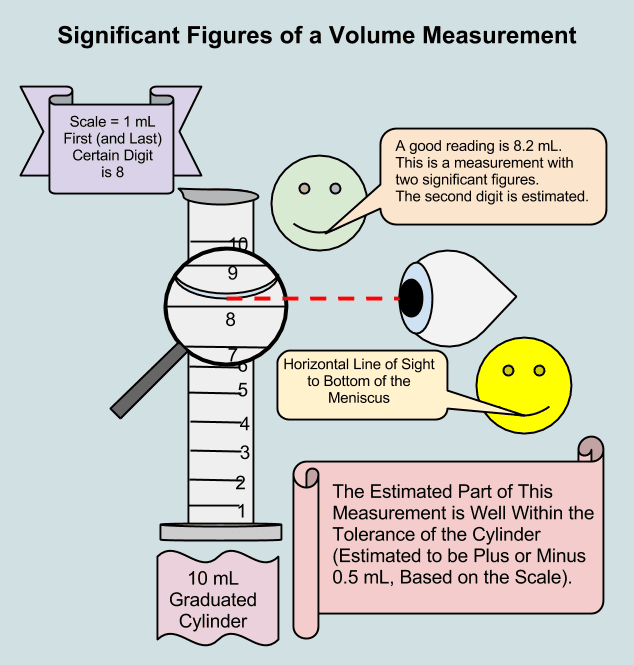
Significant Figures In Measurement
The numbers of measured quantities, unlike defined or directly counted quantities, are not exact. To measure the volume of liquid in a graduated cylinder, you should make a reading at the bottom of the meniscus, the lowest point on the curved surface of the liquid.
Refer to the illustration in Figure 1.5.1. The bottom of the meniscus in this case clearly lies between the 21 and 22 mL markings, meaning the liquid volume is certainly greater than 21 mL but less than 22 mL. The meniscus appears to be a bit closer to the 22 mL mark than to the 21 mL mark, and so a reasonable estimate of the liquids volume would be 21.6 mL. In the number 21.6, then, the digits 2 and 1 are certain, but the 6 is an estimate. Some people might estimate the meniscus position to be equally distant from each of the markings and estimate the tenth-place digit as 5, while others may think it to be even closer to the 22 mL mark and estimate this digit to be 7. Note that it would be pointless to attempt to estimate a digit for the hundredths place, given that the tenths-place digit is uncertain. In general, numerical scales such as the one on this graduated cylinder will permit measurements to one-tenth of the smallest scale division. The scale in this case has 1-mL divisions, and so volumes may be measured to the nearest 0.1 mL.
Captive zeros result from measurement and are therefore always significant. Leading zeros, however, are never significantthey merely tell us where the decimal point is located.
Don’t Miss: What Is Free Energy In Biology
What Is Repeatability With Example
Repeatability is the variation that occurs when a person uses a gage to measure the same part repeatedly with the same measurement tool. When a gage is acceptable, the variation due to repeatability is small. For example, when we measure a washer over and over again, the values should group closely together.
Example : Rounding Numbers
In the midst of all these technicalities, it is important to keep in mind the reason why we use significant figures and rounding rulesto correctly represent the certainty of the values we report and to ensure that a calculated result is not represented as being more certain than the least certain value used in the calculation.
Read Also: Is Physics Hard In High School
Difference Between Accuracy And Precision
| Basis | |
| Accurate items have to be precise in most cases. | Precise items may or may not be accurate. |
The points mentioned below are an indication of how terms like accuracy and precision vary from one another despite being used by people interchangeably on most occasions. For more information on topics like uncertainty in measurement, keep visiting BYJUS.
Key Takeaways: Accuracy Versus Precision
- Accuracy is how close a value is to its true value. An example is how close an arrow gets to the bull’s-eye center.
- Precision is how repeatable a measurement is. An example is how close a second arrow is to the first one .
- Percent error is used to assess whether a measurement is sufficiently accurate and precise.
You can think of accuracy and precision in terms of hitting a bull’s-eye. Accurately hitting the target means you are close to the center of the target, even if all the marks are on different sides of the center. Precisely hitting a target means all the hits are closely spaced, even if they are very far from the center of the target. Measurements that are both precise and accurate are repeatable and very near true values.
Also Check: How Can Biology Be Studied At Different Scales
What Is The Difference Between Accuracy And Precision
Both accuracy and precision reflect how close a measurement is to an actual value, but they are not the same. Accuracy reflects how close a measurement is to a known or accepted value, while precision reflects how reproducible measurements are, even if they are far from the accepted value. Measurements that are both precise and accurate are repeatable and very close to true values.
What Is Accuracy In Chemistry
Accuracy in chemistry refers to how close a measurement to the true value. In order to increase the accuracy, we have to calibrate the instrument that we use to take the measurement. When calibrating, we should use a proper standard as the reference.
Figure 01: Precision vs. accuracy. is neither precise nor accurate. is precise and accurate. is precise but inaccurate.
The accurate measurement should not have a systemic error or a random error. However, there are always errors occur when we take measurements from an analytical instrument, it can be either instrumental errors or human errors.
Recommended Reading: Algebra Break Even Word Problems
What Is Precision In Chemistry
Precision is defined as the extent to which results agree with one another. In other words, it is a measure of consistency, and is usually evaluated in terms of the range or spread of results. Practically, this means that precision is inherently related to the standard deviation of the repeated measurements.
Ngss Science And Engineering Practices:
AccuracyPrecision
- If the darts are neither close to the bulls-eye, nor close to each other, there is neither accuracy, nor precision .
- If all of the darts land very close together, but far from the bulls-eye, there is precision, but not accuracy .
- If the darts are all about an equal distance from and spaced equally around the bulls-eye there is mathematical accuracy because the average of the darts is in the bulls-eye. This represents data that is accurate, but not precise . However, if you were actually playing darts this would not count as a bulls-eye!
- If the darts land close to the bulls-eye and close together, there is both accuracy and precision .
You May Like: What Does N Stand For In Chemistry
Is Precision Measured By Significant Digits In Chemistry
I was recently asked this question:
An experiment is done 3 times, with a different measuring tool usedfor each time. The results of the experiment are 1.39, 0.9, and 1.0. Which measurement is the most precise, and how doyou know?
On my paper, I put that the answer was 1.39 because it had the most digits past the decimal point and submitted. However, I’ve read online and found since then that my explanation actually referred to accuracy, not precision. My question is, would saying “1.39 is the most precise because it has the most significant digits” be a correct answer?
- 5$\begingroup$The question on the test is poorly worded and of the answer you should have written is: Nobody can answer this question with the given information. If your teacher, disagrees, explain with respect that his/her answers are not correct.$\endgroup$
The single measured values provided by 3 different tools gives us just a hint about possible accuracy or precision of the tools. The tool providing the value 1.39 is probably, but not necessarily more precise and accurate than the other two.
What these values really say is just the tool resolution.
There are 4 terms:
- accuracy, also defined by ISO as trueness
- accuracy, defined by ISO as combined trueness and precision
As formulated by Wikipedia in above links:
In measurement of a set, accuracy is closeness of the measurements to a specific value, while precision is the closeness of the measurements to each other.
Summary Accuracy Vs Precision In Chemistry
Accuracy and precision are independent of each other. The difference between accuracy and precision in chemistry is that accuracy reflects how close a measurement to an accepted value whereas precision reflects how reproducible the measurements are.
Reference:
1. Helmenstine, Anne Marie. What Is Accuracy in Science? ThoughtCo. Available here 2. Helmenstine, Anne Marie. Understand the Difference Between Accuracy and Precision. ThoughtCo. Available here
Image Courtesy:
1.Precision versus accuracyBy CK-12 Foundation User:Adrignola File:High School Chemistry.pdf, page 107, via Commons Wikimedia
Don’t Miss: What Does Alpha Mean In Chemistry
Significant Figures In Calculations
A second important principle of uncertainty is that results calculated from a measurement are at least as uncertain as the measurement itself. We must take the uncertainty in our measurements into account to avoid misrepresenting the uncertainty in calculated results. One way to do this is to report the result of a calculation with the correct number of significant figures, which is determined by the following three rules for rounding numbers:
The following examples illustrate the application of this rule in rounding a few different numbers to three significant figures:
- 0.028675 rounds up to 0.0287
- 18.3384 rounds down to 18.3
- 6.8752 rounds up to 6.88
- 92.85 rounds down to 92.8
Lets work through these rules with a few examples.