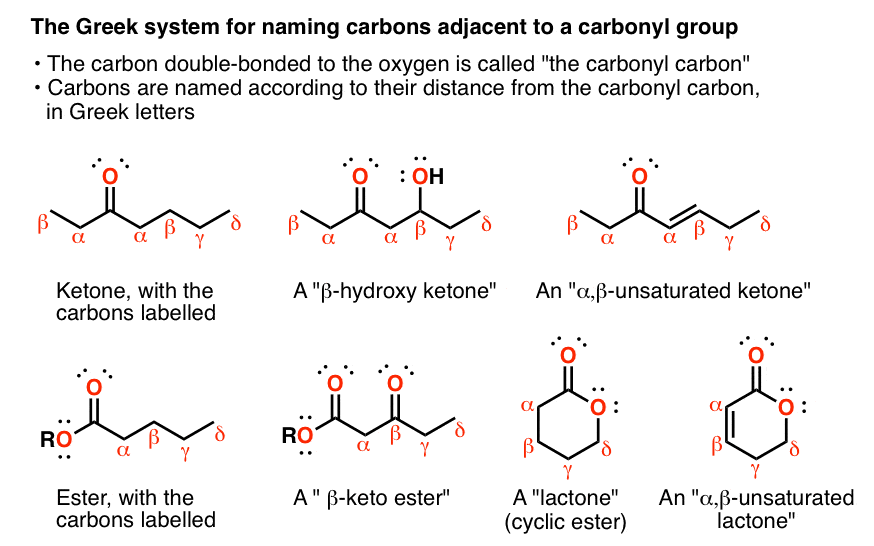
The Alpha Carbon Is The Carbon Adjacent To The Carbonyl
The functional group C=O is called a carbonyl. The carbon itself is called the carbonyl carbon, and the oxygen is called the carbonyl oxygen. But what do you call a carbon adjacent to the carbonyl carbon or 3 carbons away?
- In organic chemistry, its common to use Greek letters to denote this. So the carbon adjacent to a carbonyl is called an carbon, two carbons away is called a carbon, and so on.
- This nomenclature can be used to depict different kinds of substituted carbonyl groups. For example a ketone with an OH on the beta carbon would be called a -hydroxy ketone. If it was one carbon further down it would be a hydroxy ketone.
- If we have a double bond between the carbon and the carbon its common to call it ,-unsaturated. So we can have , unsaturated ketones, aldehydes, esters, and so on.
- It can keep going beyond gamma, of course, but its rare to see it progress beyond .
- Another thing: aldehydes, esters, carboxylic acids, and so on, can only have one alpha carbon each, wheras ketones can have two. Sometimes youll see one set of Greek symbols marked with symbols to distinguish them. The location of the prime is completely arbitrary.
- For esters, the OR group is not denoted alpha. Its usually just called the alkoxy group.
Religion And The Alpha Letter
Due to its association with the beginning of things, alpha has been very symbolically important in many religions of human history. In the creed of the Catholic religion, particularly, Alpha and Omega is a reference to Jesus Christ, as God and in association with the beginning and end of creation.
These religious connotations of the alpha letter are also notable in religious music, where they are very recurring in reference to God as the beginning of things .
Examples Of Alpha In A Sentence
alphaalpha Arkansas Onlinealpha Forbesalpha Fortunealpha Detroit Free Pressalpha USA TODAYalpha New York Timesalphasun-sentinel.comalpha BostonGlobe.comalpha Forbesalphaclevelandalphaclevelandalpha Popular Sciencealpha New York TimesalphaclevelandalphaclevelandalphaDetroit Free Press
These example sentences are selected automatically from various online news sources to reflect current usage of the word ‘alpha.’ Views expressed in the examples do not represent the opinion of Merriam-Webster or its editors. Send us feedback.
Don’t Miss: Is Paris Michael Jackson Biological Child
And 14 Additions To Alpha Beta Unsaturated Carbonyls
Another item of confusion are the terms 1,2-addition and 1,4-addition. This originates in the discussion of hydrogen halides to alkenes and dienes. Usually, addition to straight alkenes is just called addition. But when two or more alkenes are next to each other, at least two different products become possible. The numbers 1,2- and 1,4- are used to distinguish the two products from each other.
Using HBr as an example, in the first case, were forming C-H on carbon 1 and C-Br on carbon 2. Hence, 1,2-addition.
In the second reaction, we form C-H on carbon 1 and C-Br on carbon 4. Hence, 1,4-addition.
The same analogy holds for additions to carbonyls and to alpha-beta unsaturated carbonyls, but its a little bit confusing because the 1 in this case refers to oxygen: not an atom were used to numbering.
But if you can get over that little bit of weirdness, the analogy to 1,2-addition and 1,4-addition with alkenes is dead on. As an example, the 1,2-addition of water to an , unsaturated ketone gives a C-OH bond on position 2 and an O-H bond on position 1 the 1,4-addition of water to the same ketone forms C-OH on position 4 and O-H on position 1.
, its possible to have 1,6-additions. Rare, but it can happen.)
Glossary Definition Of Atomic Number
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
The atomic number of a chemical element is the number of protons in the nucleus of an atom of the element. It is the charge number of the nucleus since neutrons carry no net electrical charge. The atomic number determines the identity of an element and many of its chemical properties. The modern periodic table is ordered by increasing atomic number.
You May Like: Does Kamala Harris Have Any Biological Children
Relationship Between Atomic Number And Chemical Properties
The reason the atomic number determines the chemical properties of an element is that the number of protons also determines the number of electrons in an electrically neutral atom. This, in turn, defines the electron configuration of the atom and the nature of its outermost or valence shell. The behavior of the valence shell determines how readily an atom will form chemical bonds and participate in chemical reactions.
Physical Characteristics Of Ionizing Particulate Radiation
Of the various ionizing particulate radiations, the most important in terms of likelihood for human exposure are alpha particles, beta particles, protons, and neutrons. Alpha and beta particles occur as a result of the radioactive decay of unstable atoms. Neutrons generally result from nuclear reactions, such as nuclear fission and charged-particle activation of target atoms . Protons arise from atomic interactions of neutrons.
Alpha Particles
Alpha particles have a positive charge and are identical with helium nuclei and consist of two protons and two neutrons. They result from the radioactive decay of heavy elements such as radium, thorium, uranium, and plutonium. Because of their double-positive charge, alpha particles have great ionizing power, but their large mass results in very little penetration. For example, alpha particles from 4 to 10 MeV have ranges in air of 5â11 cm the corresponding range for alpha particles in water would be from 20 to 100 μm.
Beta Particles
Neutrons
Read Also: Geometry Assignment Find The Length Indicated Answer Key
What Is Alpha Decay
Alpha decay or -decay is a type of radioactive decay in which the atomic nucleus emits an alpha particle thereby transforming or decaying into a new atomic nucleus. Here the atomic mass number of the newly formed atom will be reduced by four and the atomic number will be reduced by two. The emitted alpha particle is also known as a helium nucleus. The mass of the alpha particles is relatively large and has a positive charge.
Ernest Rutherford distinguished alpha decay from other forms of radiation by studying the deflection of the radiation through a magnetic field. The deflection of alpha decay would be a positive charge as the particles have a +2e charge.
Gamow Theory Of Alpha Decay
The GeigerNuttall law or GeigerNuttall rule relates to the decay constant of a radioactive isotope with the energy of the alpha particles emitted. This relation also states that half-lives are exponentially dependent on decay energy, so that very large changes in half-life make comparatively small differences in decay energy, and thus alpha particle energy.
As per this rule, short-lived isotopes emit more energetic alpha particles than long-lived ones. This law was stated by Hans Geiger and John Mitchell Nuttall in the year 1911, hence the name was dedicated to these physicists.
Read Also: Ccl4 Geometric Shape
Radioactive Nuclei Emit Three Types Of Radiations
Physicists have called the three types of radiations emitted by nuclei, alpha, beta and gamma, the three first letters of the greek alphabet.
Map of decay modes
This naming convention of the three types of radiation has been in use since their discovery, and still applies today. The ancient greek alphabet was familar to physicists nourished by classical culture.Alpha radiation is the name for the emission of an alpha particle in fact an helium nuclei, beta radiation is the emission of electrons or , and gamma radiation is the term used for the emission of energetic photons. When uranium salts were found in 1896 to produce unknown emissions, two types of radiation, X-rays and cathode rays, have been just discovered. At that time, nuclei, electrons and photons were unknown. It would take decades before the origins of all these rays were properly understood, but a few years to identify their nature. Incidentally cathode rays and X-rays were found to be electrons and photons like beta and gamma radiations.
Disintegration diagramstrongweakelectromagnetic
New Elements And Atomic Numbers
At the time of this writing, elements with atomic numbers 1 through 118 have been identified. Scientists typically talk about discovering new elements with higher atomic numbers. Some researchers believe there may be an “island of stability,” where the configuration of protons and neutrons of superheavy atoms will be less susceptible to the quick radioactive decay seen in known heavy elements.
Don’t Miss: What Does Abiotic Mean In Biology
Alpha Symbol Maths And Science
The letter alpha represents various concepts in physics and chemistry, including alpha radiation, angular acceleration, alpha particles, alpha carbon, and electromagnetic interaction force.
It also means a coefficient of thermal expansion of a compound in physical chemistry. It is commonly used in mathematics in algebraic solutions that represent quantities such as angles. Furthermore, it is used to denote the area under a normal curve in the statistics to denote the level of significance when testing null and alternative hypotheses.
In zoology, it is used to name the dominant individual in a pack of wolves or dogs. In aerodynamics, the letter is used as a symbol of the angle of attack of an airplane and the word alpha is used as a synonym for this property. The capital letter alpha is not generally used as a symbol because it tends to be identical to the Latin capital letter A.
Alpha Particles And Protons
Properties of alpha particles and protons are listed in Table 1.1. Strong coulombic interactions lead to the ionization and excitation of the atoms in the target material along the tracks made by the alpha and proton particles. In physics, the target material is usually some inanimate material, whereas in radioecology, the target often refers to plant or animal tissue. The rate at which energy is dissipated in the target material is known as the stopping power in nuclear physics and the linear energy transfer in radiobiology. Alpha particles have a high LET, meaning that all of their energy is absorbed by the biological tissue over a very short distance. The particles do not move in straight lines, but exhibit a circuitous path influenced by their interactions with the target material. LET data for different classes of 1 MeV radiation in water are listed in Table 1.3. The projected range for 1 and 5 MeV alphas in water are 0.006 and 0.037 mm, respectively.
Table 1.3. Interaction of Radiation with Water
| Radiation |
|---|
P. Falkner, R. Schulz, in, 2015
You May Like: Who Are Paris Jackson’s Biological Parents
Proteins And Amino Acids
Alpha-carbon is also a term that applies to proteins and amino acids. It is the backbone carbon before the carbonyl carbon atom in the molecule. Therefore, reading along the backbone of a typical protein would give a sequence of n etc. . The -carbon is where the different substituents attach to each different amino acid. That is, the groups hanging off the chain at the -carbon are what give amino acids their diversity. These groups give the -carbon its stereogenic properties for every amino acid except for glycine. Therefore, the -carbon is a stereocenter for every amino acid except glycine. Glycine also does not have a -carbon, while every other amino acid does.
The -carbon of an amino acid is significant in protein folding. When describing a protein, which is a chain of amino acids, one often approximates the location of each amino acid as the location of its -carbon. In general, -carbons of adjacent amino acids in a protein are about 3.8 ångströms apart.
The Alpha Letter In Biology
In the field of biology , an individual of a species that plays the role of leader of the pack, is called the alpha individual, having privileges as regards to feeding and mating.
Normally, the other copies of the herd follow the leader and regularly show signs of obedience, although there are cases where the leader is challenged and defeated, after which there is a new alpha individual. This concept is only applicable for animal species that live in packs, such as wolves, lions, etc.
Recommended Reading: Who Is Paris Jacksons Biological Father
Alpha Symbol: Origins And Their Meaning
If we are going to examine the meaning of the alpha symbol, we should definitely start by explaining what the phrase Alpha and Omega means. After all, this religious context is the most prominent and popular use of both symbols. God declares his eternal nature and that he is the beginning and the end of everything that exists using the first and last letters of the Greek alphabet.
Therefore, it could be said that the concepts of emet and alpha and omega used to represent God in two of the most prominent religions in the world are similar in this sense. The alpha and omega symbols/letters were frequently used in early Christian art and sculpture, in addition to being inscribed on crosses used in those times.
These symbols were often used in conjunction with chi and rho, the first two letters of the word Christ in the Greek language .
What Does Alpha Radiation Mean
Alpha radiation is a type of ionizing radiation that consists of emitted alpha particlespositively charged particles that consist of two protons and two neutrons. Alpha particles are the largest and most highly charged form of radiation energy.
Alpha radiation has little penetration ability, and is a low-risk to workers as a source of external exposure however, it is a significant health hazard if ingested, inhaled, or absorbed through broken skin.
Don’t Miss: Definition Of Abiotic In Science
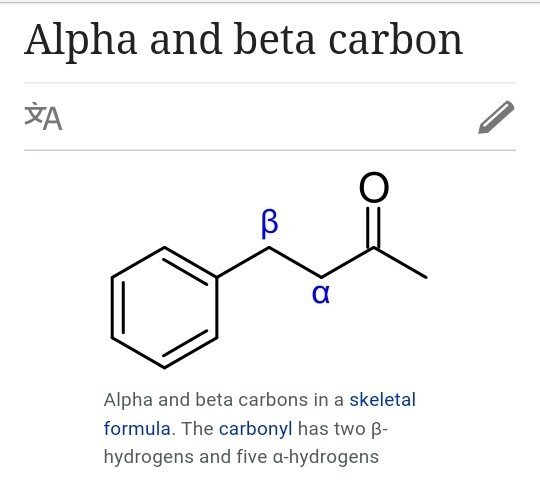
Alpha And Beta Carbon
The alpha carbon in organic molecules refers to the first carbonatom that attaches to a functional group, such as a carbonyl. The second carbon atom is called the beta carbon , and the system continues naming in order with Greek letters.
The nomenclature can also be applied to the hydrogen atoms attached to the carbon atoms. A hydrogen atom attached to an alpha carbon atom is called an alpha-hydrogen atom, a hydrogen atom on the beta-carbon atom is a beta hydrogen atom, and so on.
This naming standard may not be in compliance with IUPAC nomenclature, which encourages that carbons be identified by number, not by Greek letter, but it nonetheless remains very popular, in particular because it is useful in identifying the relative location of carbon atoms to other functional groups.
Organic molecules with more than one functional group can be a source of confusion. Generally the functional group responsible for the name or type of the molecule is the ‘reference’ group for purposes of carbon-atom naming. For example, the molecules nitrostyrene and phenethylamine are quite similar the former can even be reduced into the latter. However, nitrostyrene’s -carbon atom is adjacent to the phenyl group in phenethylamine this same carbon atom is the -carbon atom, as phenethylamine counts its atoms from the opposite “end” of the molecule.
-
Nitrostyrene
Safeopedia Explains Alpha Radiation
Alpha radiation has a lower kinetic energy than any other form of common radiation. The large size of alpha particles restricts them to traveling only a few centimeters through air and their positive charge allows them to be easily shielded against. If alpha radiation makes contact with a human it will not penetrate further than the outer layers of human skin, and is normally stopped by dead skin cells. When alpha radiation is emitted inside the body, however, the large size and positive charge of alpha particles make alpha radiation the most damaging type.
The most significant source of hazardous alpha radiation is radon gas, which decays into alpha radiation-emitting radon daughters. Radon daughters can attach to atmospheric dust and water droplets which can be breathed into the lungs and airways. This is a major risk factor for lung cancer. OSHA recommends that personal protective equipment should be used where high levels of alpha radiation are found in order to prevent external contamination with material containing inhalable alpha radiation emitters.
Recommended Reading: Ccl4 Lone Pairs
Alpha Beta And Gamma Radiation
Emissions from radioactive nuclei are called, collectively, ionizing radiation because collision between these emissions and an atom or molecule ionizes that atom or molecule. Ionizing radiation may be characterized further as alpha, beta, or gamma radiation by its behavior in a magnetic field. Apparatus for such characterization is shown in Figure 16-2. A beam of radioactively disintegrating atoms is aimed with a lead barrel at a fluorescent screen that is designed to glow when hit by the radiation. Alternately charged probes direct the α and β radiation accordingly. The γ radiation is seen to be âinvisible light,â a stream of neutral particles that passes undeflected through the electromagnetic field, α and β emissions have some mass and are considered particles, while γ emissions are photons of electromagnetic radiation.
FIGURE 16-2. Controlled measurement of alpha , beta , and gamma radiation
TABLE 16-2. Properties of Ionizing Radiation
| Particle or Photon |
|---|
When a radionuclide emits a β, the mass number remains unchanged and the atomic number increases by 1 . When a nuclide emits an α, the atomic mass decreases by 4 and the atomic number decreases by 2. γ emission does not result in a change of either atomic mass or atomic number.
Nuclear reactions may also be written for bombardment of nuclei with subatomic particles. For example, tritium is produced by bombarding a lithium target with neutrons:
Peter Airey, … John Twining, in, 2012
Alpha Symbol In Greek Alphabet
symbolsalphasymbol
Like the other Greek letters, alpha is commonly used in chemistry, engineering, physics, mathematics, and science. Etymologically, alpha came from aleph (the first letter…
IPA: /a/
Letter
1. The first letter of the ArvaniticAlbanian Greek-script alphabet.
See also
1. , , , , j j, , D d, , , , , , , J j, , , , , , , , , , , , , , , , , , , , , , , D d, D d 2. shkronjë A a, B b, C c, Ç ç, D d, Dh dh, E e, Ë ë, F f, G g, Gj gj, H h, I i, J j, K k, L l, Ll ll, M m, N n, Nj nj, O o, P p, Q q, R r, Rr rr, S s, Sh sh, T t, Th th, U u, V v, X x, Xh xh, Y y, Z z, Zh zh
Etymology 1
Derived from its Ancient Greek majuscule counterpart , in turn from the Phoenician letter .
Etymology 2
From Ancient Greek .
Etymology 3
From < from Ancient Greek sg of verb . Also see etymologies of , , .
Read Also: Geometry Segment Addition Postulate Worksheet