Replacing The Water In The Hexaaquachromium Ion
Again, all the agua ligands are replaced by ammine ligands. The difference this time is that the reaction is incomplete. The precipitate has to be left to stand in the presence of excess concentrated ammonia solution for some time to get the fully substituted ammine complex. Even so, you still get left with some unreacted precipitate.
\^ + 6NH3 < => ^ + 6H2O}\nonumber \]
Strong Field And Weak Field Ligands
In general, ligands are viewed as electron donors and the metals as electron acceptors, i.e., respectively, Lewis bases and Lewis acids. This description has been semi-quantified in many ways, e.g. ECW model. Bonding is often described using the formalisms of molecular orbital theory.
Ligands and metal ions can be ordered in many ways one ranking system focuses on ligand ‘hardness’ . Metal ions preferentially bind certain ligands. In general, ‘hard’ metal ions prefer weak field ligands, whereas ‘soft’ metal ions prefer strong field ligands. According to the molecular orbital theory, the HOMO of the ligand should have an energy that overlaps with the LUMO of the metal preferential. Metal ions bound to strong-field ligands follow the Aufbau principle, whereas complexes bound to weak-field ligands follow Hund’s rule.
Binding of the metal with the ligands results in a set of molecular orbitals, where the metal can be identified with a new HOMO and LUMO and a certain ordering of the 5 d-orbitals . In an environment, the 5 otherwise degenerate d-orbitals split in sets of 2 and 3 orbitals .
- 3 orbitals of low energy: dxy, dxz and dyz
- 2 of high energy: dz2 and dx2y2
For complexes with a tetrahedral surrounding, the d-orbitals again split into two sets, but this time in reverse order.
- 2 orbitals of low energy: dz2 and dx2y2
- 3 orbitals of high energy: dxy, dxz and dyz
Design Of Ldai Reagents For Gabaar
LDAI reagents consist of four modules: an acyl imidazole moiety as the cleavable reactive group, a ligand moiety having sufficient affinity and selectivity for the target protein, a spacer moiety between acyl imidazole and the ligand, and a fluorophore group. For chemical labeling to GABAAR, we prepared three LDAI reagents , which are targeted for the orthosteric GABA-, allosteric benzodiazepine-, or barbiturate-site, respectively . As a typical example, we here describe how to design a LDAI reagent for benzodiazepine site in detail.
Renjie Huang, Ivanhoe K.H. Leung, in, 2019
Read Also: Exponent Rules Worksheet Algebra 2
What Is A Ligand
Do You Know the Ligand Definition?
A ligand is an ion or molecule that binds to a central metal atom to form a coordination complex in coordination chemistry. Formal donation of one or more of the ligand’s electron pairs, often via Lewis Bases, is necessary for bonding with the metal. Metal ligand bonding can be either covalent or ionic in nature. The metal-ligand bond order can also vary from one to three. Lewis bases are considered ligands, while Lewis acidic “ligands” have been found in rare cases.
As we already defined ligands, now lets take a look at examples of neutral ligands , cationic ligands, and neutral ligands.
Replacing Water Molecules By Ammonia
Water molecules and ammonia molecules are very similar in size, and so there is no change in co-ordination this time. Unfortunately, the reactions aren’t quite so straightforward to describe. Ammonia solution can react with hexaaqua metal ions in two quite distinct ways, because it can act as a base as well as a ligand.
If you add a small amount of ammonia solution you get precipitates of the metal hydroxide – the ammonia is acting as a base. In some cases, these precipitates redissolve when you add more ammonia to give solutions in which a ligand exchange reaction has occurred. In the diagrams below, both steps are shown, but we are only going to consider the chemistry of the overall ligand exchange reaction. The precipitates dissolve because of a complicated series of equilibrium shifts, and we shan’t worry about that for the moment.
Read Also: My Hrw Com Algebra 1
Replacing The Water In The Hexaaquacobalt Ion
This time, all the water molecules get replaced.
\^ + 6NH3 < => ^ + 6H2O}\nonumber \]
The straw colored solution formed changes color very rapidly on standing to a deep reddish brown. The hexaamminecobalt ions are oxidised by the air to hexaamminecobalt ions. However, that is a quite separate reaction, and is not a part of the ligand exchange reaction.
Synthesis And Reactivity Of Metal Complexes With Multifunctional Noninnocent Ligands
We are designing and investigating redox-active ligands that also play an active role in substrate binding via metal-ligand cooperative reactions. The goal is to couple both types of ligand-centered reactivity to promote new catalytic transformations, and fundamental studies are aimed at identifying how chemical binding at redox-active ligands affects their redox properties.
Recent Publications: Spielvogel, K. D. * Luna, J. A. * Loria, S. M. Weisburn, L. P. Stumme, N. C. Ringenberg, M. R. Durgaprasad, G. Keith, J. M. Shaw, S. K. Daly, S. R. “The Influence of Multisite Metal-Ligand Cooperativity on the Redox Activity of Noninnocent N2S2 Ligands.” Inorg. Chem.2020, 59, 1084510853 . *Equal contributions.
Spielvogel, K. D. Coughlin, E. J. Petras, H. Luna, J. A. Benson, A. Donahue, C. M. Kibasa, A. Lee, K. Salacinski, R. Bart, S. C. Shaw, S. K. Shepherd, J. Daly, S. R. “The Influence of Redox-Innocent Donor Groups in Tetradentate Ligands Derived from o-Phenylenediamine: Electronic Structure Investigations with Nickel.” Inorg. Chem.2019, 58, 12756-12774 .
Durgaprasad, G Luna, J., A. Spielvogel, K. D. Haas, C. Shaw, S. K. Daly, S. R. “Ru Complexes with a Chemical and Redox-Active S2N2 Ligand: Structures, Electrochemistry, and Metal-Ligand Cooperativity.” Organometallics2017, 36, 4020-4031
Also Check: Evaluating Functions Worksheet Algebra 2 Answer Key
Ligating Power Of Pyridine
The spectrochemical series of ligands in crystal field theory , portray ligand arrangement pertaining to their metal d-orbital splitting capability, depicts pyridine as moderately strong ligand . This interprets to strong electrostatic interactions of pyridine lone pair to metal d- orbitals. Despite being neutral, pyridine causes moderately large d-orbital splitting implying to strong bonding interaction to metal centers. Beside the CFT, the valence bond theory , considers metal pyridine bonding to overlap of sp2 lone pair orbital of pyridine to hybridized metal orbitals. The extent of overlaps is happened to be highest in first transition metals in comparison to the second and third transition elements owing to the difference of shape, size, and energy of the combining orbitals. Apart from nitrogen lone pair orbitals, the ring -electron is also capable of bonding interaction to metal ions. Moreover delocalized * anti-bonding orbitals can act as acceptor of metal electron density . The pyridine can also indulge in hydrogen bonding and – stacking-like weak interactions. Thus pyridine is enriched with multiple orbitals for bonding interactions with metal ions.
Figure 2.
Bonding picture of pyridine.
Protein And Ligand Concentrations
Ligand concentration: WaterLOGSY typically detects ligands that bind with a KD in the high M to low mM region. In order to ensure the binding of these low- to medium-affinity ligands, a ligand molar excess of 25- to 100-fold is usually used.
As discussed earlier, the waterLOGSY response of a protein ligand may be positive, or less negative than the corresponding signal in the control spectrum minus the protein. Whether positive or less negative waterLOGSY responses are observed depends on both the binding affinity of the ligand and the ligand molar excess . For weaker binders, a large molar excess would push the binding equilibrium toward the proteinligand complex and hence increases the population that exhibit negative NOE signals , while for stronger binders, a large molar excess merely increases the free population of the ligand and hence decreases the population that exhibit negative NOEs . In cases as such, a lower ligand molar excess may enable the clearer, positive waterLOGSY signals to be detected.
F. Wang, X. Liu, in, 2011
Recommended Reading: Why Are There Different Branches Of Chemistry
The Naming Of Complexes
The nomenclature of the complexes is patterned after a system suggested by Alfred Werner, a Swiss chemist and Nobel laureate, whose outstanding work more than 100 years ago laid the foundation for a clearer understanding of these compounds. The following five rules are used for naming complexes:
| Anionic Ligand |
|---|
Draw the ion trans-diaqua-trans-dibromo-trans-dichlorocobalt.
Difference Between Double Salts And Coordination Compounds
Those compounds which lose their identity in solution are called double salts. when FeSO4. SO4. 6H2O dissolved in water the parts of molecules are separated from each other. The compounds are dissociated in water to give simple ions.
FeSO4. SO4. 6H2O Fe2+ + 2NH4+ + 2SO42- + 6H2O
KCl. MgCl2. 6H2O , K2SO4. Al23. 24H2O are examples of double salts.
Those compoundsthat do not lose their identity in solution are called coordination or Complex compounds.
K4 4K+ + 4-
4- is a complexion that does not give the test of Fe2+ ion. SO4, K are examples of complex compounds.
Don’t Miss: Do You Capitalize Bachelor’s Degree In Psychology
What Are Different Types Of Ligands
A ligand is an ion or molecule, which donates a pair of electrons to the central metal atom or ion to form a coordination complex. The word ligand is from Latin, which means tie or bind. Ligands can be anions, cations, and neutral molecules. Ligands act as Lewis bases and central metal atoms viewed as Lewis acid . The nature of bonding between metal to ligand varies from covalent bond to ionic bond.
Occasionally ligands can be cations and electron-pair acceptors. Examples for anionic ligands are F, Cl, Br, I, S2, CN, NCS, OH, NH2 and neutral ligands are NH3, H2O, NO, CO.
A ligand is an ion or molecule, which binds to the central metal atom to form a coordination entity or complex compounds. Classification of ligands is on the basis of the number of binding sites with the central metal atom, charge and size.
Also Read:Coordination compound
Coordination Complexes In Nature And Technology
Chlorophyll, the green pigment in plants, is a complex that contains magnesium . This is an example of a main group element in a coordination complex. Plants appear green because chlorophyll absorbs red and purple light the reflected light consequently appears green. The energy resulting from the absorption of light is used in photosynthesis.
Figure 14.
You May Like: What Is Figure Ground Perception Psychology
Jee Chemistry Ligands And Its Types And Co
Co-ordination ChemistryCo-ordination CompoundsLigand
Types of ligands
| Monodentate Ligand |
Replacement Of The Water By Chloride Ions
In the presence of chloride ions chloride), the most commonly observed color is green. This happens when two of the water molecules are replaced by chloride ions to give the tetraaquadichlorochromium ion – +. Once again, notice that replacing water molecules by chloride ions changes the charge on the ion.
Don’t Miss: How To Intimidate Someone Psychologically
Key Concepts And Summary
The transition elements and main group elements can form coordination compounds, or complexes, in which a central metal atom or ion is bonded to one or more ligands by coordinate covalent bonds. Ligands with more than one donor atom are called polydentate ligands and form chelates. The common geometries found in complexes are tetrahedral and square planar and octahedral . Cis and trans configurations are possible in some octahedral and square planar complexes. In addition to these geometrical isomers, optical isomers are possible in certain octahedral complexes. Coordination complexes have a wide variety of uses including oxygen transport in blood, water purification, and pharmaceutical use.
Ligand Definition In Chemistry
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
A ligand is an atom, ion, or molecule that donates or shares one or more of its electrons through a covalent bond with a central atom or ion. It is a complexing group in coordination chemistry that stabilizes the central atom and determines its reactivity. Ligands are usually considered to be Lewis bases, although a few cases of Lewis acid ligands exist.
Some sources only consider ligands to be functional groups that bind to a central metal complex. In these cases, the bonds formed within the ligand may range from covalent to ionic in nature.
Read Also: Span Of Vectors
Uses Of Dimethylglyoxime C4h8n2o2
- Widely used in analytical chemistry as a selective precipitating reagent, detecting reagent and photometric reagent for nickel, palladium, platinum and some other metal ions.
- Used as a test for nickel release and used for jewellery and for other objects that come in direct contact with the skin. In many countries the dimethylglyoxime test is now commercially available in pharmacies or chemist shops.
- Used as a specific precipitant for nickel and palladium. Nickel precipitates as a bright red voluminous compound from ammoniacal solution, white palladium come down as a yellow compound from dilute hydrochloric acid solutions. These are dried and weighed as stoichiometric compounds.
Pyridine Transition Metal Complexes
The pyridine transition metal complexes have a rich literature. Pyridine found to coordinate all the transition metals producing the variety of metal complexes in their different oxidation states. Efforts were made to incorporate the increasing number of pyridines in metal coordination sphere but exclusive pyridine complexes such as n+ or n+ are rare . The metal- pyridine chemistry incorporates pyridine and its derivatives with the capability of bidentate or tridentates ligands in the formation of metal complexes. The discussion here to be restricted to the domain of pyridine and it coordination to transition metals. A brief overview of pyridine transition metals complexes is presented here.
Scandium and yttrium preferably bind to three pyridine units in a four-coordinated geometry. The coordination number might vary with characteristics of binding ligands. Five and six coordinated complexes are also synthesized in combination of pyridine and thiocyanate ligands. The pyridine derivative capable of acting as a bidentate ligand, such as picolinic acid, prefers to produce higher coordination number complexes. A good number of complexes are known with variously substituted pyridines. These complexes are known in +1 and + 3 states of Sc and Y. The example of pyridine coordinated complexes include , , , and . These complexes could be derived directly from their metal salt and pyridine at room temperature.
Figure 3.
Representative pyridine complexes of Ti, V and Nb.
Figure 4.
Read Also: Which Theories Of Motivation Has Biological Orientation
What Gets Stored In A Cookie
This site stores nothing other than an automatically generated session ID in the cookie no other information is captured.
In general, only the information that you provide, or the choices you make while visiting a web site, can be stored in a cookie. For example, the site cannot determine your email name unless you choose to type it. Allowing a website to create a cookie does not give that or any other site access to the rest of your computer, and only the site that created the cookie can read it.
Chemical Properties Of Dimethylglyoxime C4h8n2o2

- Nickel cation reacts with dimethylglyoxime forms an insoluble red precipitate of nickel dimethylglyoxime.
Ni2+ + 2C4H8N2O2 Ni2 + 2H+
- Dimethylglyoxime reacts with ferrous sulphate and ammonium hydroxide forms a complex compound of iron and ammonium sulphate and water is formed.
FeSO4 + 2NH4OH + 2C4H8N2O2 Fe2 + 2SO4 + 2H2O
Recommended Reading: Define Cardinal Directions
What Is Bipy In Chemistry
4.1/5Bipyridinebipyridine
Also, is Bpy neutral?
Bipyridine Is A Neutral Chelating Ligand Tha
Beside above, what shape is Fe BIPY 3 2+? 2+ is octahedral, containing a central low spin d6 Ru ion and three bidentate bpy ligands. The complex is chiral, with D3 symmetry. It has been resolved into its enantiomers, which are kinetically stable.
In respect to this, is BIPY a pi acceptor?
cyanogen, NC-CN). Charge donation into such systems results in stabilization, therefore bipyridine is found among the pi–acceptor ligands.
Is pyridine a neutral ligand?
A ligand can be an anion or a neutral molecule that donates an electron pair to the complex . Rule 2: Neutral Ligands.
Application Of Chelate Compound
- Vitamin B12, essential for human body, is a Cobalt complex.
- Homeoglobin in red blood cells contain porphyrin Complex and Fe2+ ion is central metal ion.
- Chlorophyll is a green plant pigment containing magnesium for porphyrin complex.
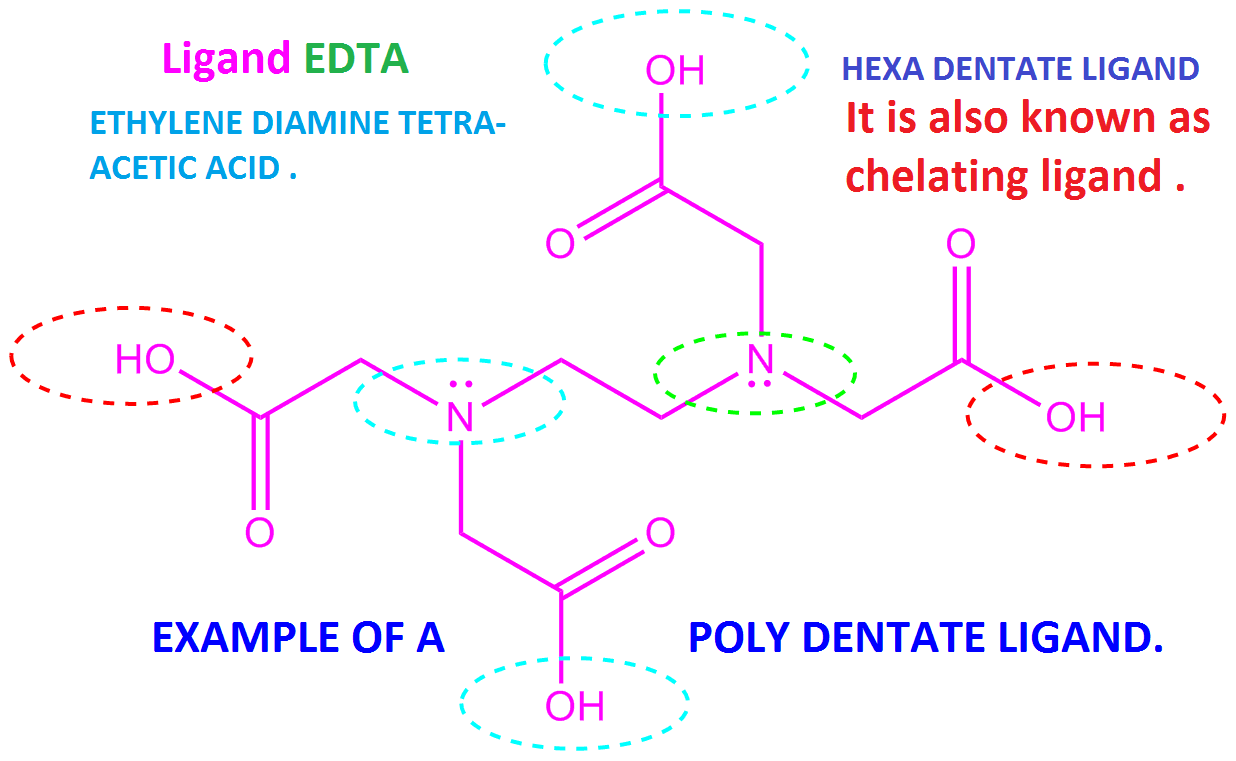
- Metal poisoning by lead copper chromium, Nickel, zinc, etc in the body can be removed by treating with EDTA. It forms a metal EDTA which is easily excreted in the urine.
2. In analytical chemistry, chelates are used in qualitative and quantitative analysis. For example, Ni2+, Mg2+, Cu2+, etc are quantitatively precipitated by the chelating agents as insoluble compounds.
3. Standard EDTA a hexadentate ligand solution is widely used in volumetric analysis to determine the hardness of the water.
Recommended Reading: Percent Error Problems Chemistry