Corrosionpedia Explains Oxidized Copper
The most obvious example of oxidation is steel corrosion, which involves the transformation of iron molecules into iron oxides, usually that of Fe2O3 and Fe3O4. This is observed on common items such as old, rusted cars, nails and metal scraps.
Similarly, when exposed to the atmosphere, copper will oxidize due to a reaction with oxygen and liquid water or moisture in the air.
The characteristic red outer layer that forms when iron corrodes is caused by oxidation. In this case, the outer layer doesn’t exactly become part of the iron but instead flakes off, thus leading to the gradual wearing away of the iron and leaving it vulnerable to structural decay and further rusting. However, in copper oxidation the oxide layer prevents oxygen exposure that would otherwise cause further corrosion. Oxidation adds a blue-green color to copper, brass and bronze.
Figure 1. Oxidized copper on a building.
The basic reaction of copper and atmospheric oxygen, which converts copper to copper oxide, is:
2 Cu + O2 –> 2 CuO
The copper to patina conversion is a multiple-step process, and it takes a long time to form copper carbonate. The oxides that can form during the oxidation process of copper to patina include:
Oxidation Reduction Reaction In Terms Of Oxidation Number
Oxidation and reduction reaction can be defined, depending on increase or decrease of oxidation number:
- Oxidation is an increase of oxidation number.
- Reduction is a decrease of oxidation number.
As for example a reaction between cupper oxide and magnesium is an oxidation reduction reaction. Here the oxidation number of Magnesium increases from 0 to +2. So this is an oxidation process. On other hand Cupper ion reduced to Cupper. The oxidation number decreases from +2 to 0.
Metals That Resist Oxidation
Noble metals, such as platinum or gold, resist oxidation in their natural state. Other such metals include ruthenium, rhodium, palladium, silver, osmium, and iridium. Many corrosion-resistant alloys have been invented by humans, such as stainless steel and brass.
While one would think that all metals that resist oxidation would be deemed noble metals, that’s not the case. Titanium, niobium, and tantalum all resist corrosion, but they’re not classified as noble metals. In fact, not all branches of science agree on the definition of noble metals. Chemistry is more generous with its definition of noble metals than physics, which has a more limited definition.
Metals that resist oxidation are the opposite of the metals prone to it, known as the base metals. Examples of base metals include copper, lead, tin, aluminum, nickel, zinc, iron, steel, molybdenum, tungsten, and other transitional metals. Brass and bronze, and the alloys of these metals, also are classified as base metals.
Don’t Miss: Theory Of Everything Geometry Dash 2
Chemistry/reduction And Oxidation Reactions
Reduction is the loss of oxygen atom from a molecule or the gaining of one or more electrons.A reduction reaction is seen from the point of view of the molecule being reduced, as when one molecule gets reduced another gets oxidised. The full reaction is known as a Redox reaction. This is a good way of remembering it.
This can be remembered with the term OIL RIG when speaking about electrons.
- Oxidation Is Loss of electrons
- Reduction Is Gain of electrons
In the case of Organic Chemistry it is usually the case of the gaining/loss of Oxygen/Hydrogen
In Inorganic Chemistry the term refers to the change in oxidation state of the metal center.
- Oxidation is a process where a substance:
- Loses one or more electrons
- Gains an oxygen atom or Electronegative atoms
- Loses a hydrogen atom or Electropositive atoms
- Gains an increase in its oxidation number
- Reduction is a process where a substance:
- Gains one or more electrons
- Loses an oxygen atom or Electronegative atoms
- Gains a hydrogen atom or Electropositive atoms
- Loses an increase in its oxidation number
Oxidation And Reduction In Terms Of Oxygen Transfer
The terms oxidation and reduction can be defined in terms of the adding or removing oxygen to a compound. while this is not the most robust definition, as discussed below, it is the easiest to remember.
Oxidation and Reduction with respect to Oxygen Transfer
- Oxidation is the gain of oxygen.
- Reduction is the loss of oxygen.
For example, in the extraction of iron from its ore:
Because both reduction and oxidation are occurring simultaneously, this is known as a redox reaction.
An oxidizing agent is substance which oxidizes something else. In the above example, the iron oxide is the oxidizing agent. A reducing agent reduces something else. In the equation, the carbon monoxide is the reducing agent.
- Oxidizing agents give oxygen to another substance.
- Reducing agents remove oxygen from another substance.
You May Like: Algebra 1 Eoc Practice Test 2015
Balancing Redox Equations In Acidic Solution: Basic Rules
If a reaction occurs in an acidic environment, you can balance the redox equation as follows:
What Is Oxidation In Chemistry
A good example of oxidation in chemistry is metal displacement. In a compound or solution, a metal atom replaced another metal atom. Copper, for instance, is formed when the reaction of zinc metal takes place in a solution of copper sulfate:
Zn + CuSO4 ZnSO4 + Cu
In the above-mentioned redox reaction example, in a solution of copper sulfate, copper ion is displaced by zinc metal, and free copper metal is produced. The reaction is spontaneous and produces 213 kJ per 65 g of zinc since, compared to zinc, the low energy of copper metal is because of bonding through its d-orbitals being partially filled.
The reaction for ionic equation given as:
Zn + Cu2+ Zn2+ + Cu
As two half-reactions, zinc is oxidized:
Zn Zn2+ + 2e
And the copper is reduced:
Cu2+ + 2e Cu
Nitrate reduction to nitrogen in the presence of acid known as de-nitrification is another example. The reaction can be written as:
2NO3 + 10e + 12H+ N2 + 6H2O
Hydrocarbon combustion, like internal combustion engines, produces H2O, CO, some partly oxidized sources of CO2 and heat energy. The full oxidation of carbon-containing materials creates CO2.
The oxidation of hydrocarbons in organic chemistry by oxygen forms water and subsequently, alcohol, aldehyde or a carboxylic acid, ketone, and afterward a peroxide.
You May Like: How To Find Average Speed In Physics
Redox Reactions In Industry
Cathodic protection is a technique used to control the corrosion of a metal surface by making it the cathode of an electrochemical cell. A simple method of protection connects protected metal to a more easily corroded “sacrificial anode” to act as the anode. The sacrificial metal instead of the protected metal, then, corrodes. A common application of cathodic protection is in galvanized steel, in which a sacrificial coating of zinc on steel parts protects them from rust.
Oxidation is used in a wide variety of industries such as in the production of cleaning products and oxidizing ammonia to produce nitric acid.
Redox reactions are the foundation of electrochemical cells, which can generate electrical energy or support electrosynthesis. Metal ores often contain metals in oxidized states such as oxides or sulfides, from which the pure metals are extracted by smelting at high temperature in the presence of a reducing agent. The process of electroplating uses redox reactions to coat objects with a thin layer of a material, as in chrome-platedautomotive parts, silver platingcutlery, galvanization and gold-platedjewelry.
Classical Idea Of Oxidation And Reduction Reactions:
Oxidation reactions involve:
C + O2 CO2
2. Addition of electronegative element:
Fe + S FeS
3. Removal of hydrogen:
H2S + Br2 2 HBr + S
4. Removal of electropositive elements:
2 KI + H2O2 I2 + 2 KOH
Oxidising agent is a substance which brings about oxidation. In the above examples O2, S, Cl2, Br2, and H2O2 are oxidising agents.
Reduction reactions involve:
N2 + 3 H2 2NH3
2. Addition of electropositive element:
SnCl2 + 2HgCl2 SnCl4 + Hg2Cl2
3. Removal of oxygen
ZnO + C Zn + CO
4. Removal of electronegative element
2FeCl3 + H2 2FeCl2 + 2HCl
Reducing agent is a substance which brings about reduction. In the above examples H2, HgCl2 and C are Reducing agents.
Note: A substance, which undergoes oxidation, acts as a reducing agent while a substance, which undergoes reduction, acts as an oxidising agent.
You May Like: Kuta Angle Addition Postulate
Setting Your Browser To Accept Cookies
There are many reasons why a cookie could not be set correctly. Below are the most common reasons:
- You have cookies disabled in your browser. You need to reset your browser to accept cookies or to ask you if you want to accept cookies.
- Your browser asks you whether you want to accept cookies and you declined. To accept cookies from this site, use the Back button and accept the cookie.
- Your browser does not support cookies. Try a different browser if you suspect this.
- The date on your computer is in the past. If your computer’s clock shows a date before 1 Jan 1970, the browser will automatically forget the cookie. To fix this, set the correct time and date on your computer.
- You have installed an application that monitors or blocks cookies from being set. You must disable the application while logging in or check with your system administrator.
How To Calculate Oxidation Number
To calculate oxidation number we need to understand and follow certain rules. There are six rules:
- Each atom in an element either in its free or uncombined state holds up an oxidation number of zero. Clearly, each atom in H2, Cl2, P4, Na, Al, O2, O3, S8, and Mg, has an oxidation number zero.
- The oxidation number of ions which comprise of only one atom is equal to the actual charge on the ion.
- In most of the compounds, the oxidation number of oxygen is 2. There are two exceptions here.
- Peroxides- Every oxygen atom is allocated an oxidation number of 1. Example, Na2O2
- Superoxides- Every oxygen atom is allocated an oxidation number of . Example, KO2
- Oxygen is bonded to fluorine- Example, dioxygen difluoride where the oxygen atom is allocated an oxidation number of +1.
Also Check: Is Psychology Major Capitalized
When Does Oxidation Occur
This chemical process can occur either in the air or after the metal is exposed to water or acids. The most common example is the corrosion of steel, which is a transformation of the iron molecules on steel’s surface into iron oxides, most often Fe2O3 and Fe3O4.
If you’ve ever seen an old, rusted car or rusted pieces of metal scraps, you’ve seen oxidation at work.
Examples Of Redox Reactions
In the reaction between hydrogen and fluorine, hydrogen is being oxidized and fluorine is being reduced:
- H2 + F2 2 HF
This reaction is spontaneous and releases 542 kJ per 2 g of hydrogen because the H-F bond is much stronger than the weak, high-energy F-F bond. We can write this overall reaction as two half-reactions:
the oxidation reaction:
and the reduction reaction:
- F2 + 2 e 2 F
Analyzing each half-reaction in isolation can often make the overall chemical process clearer. Because there is no net change in charge during a redox reaction, the number of electrons in excess in the oxidation reaction must equal the number consumed by the reduction reaction .
Elements, even in molecular form, always have an oxidation state of zero. In the first half-reaction, hydrogen is oxidized from an oxidation state of zero to an oxidation state of +1. In the second half-reaction, fluorine is reduced from an oxidation state of zero to an oxidation state of 1.
When adding the reactions together the electrons are canceled:
-
H
Read Also: Holt Geometry Lesson 4.5 Practice B Answers
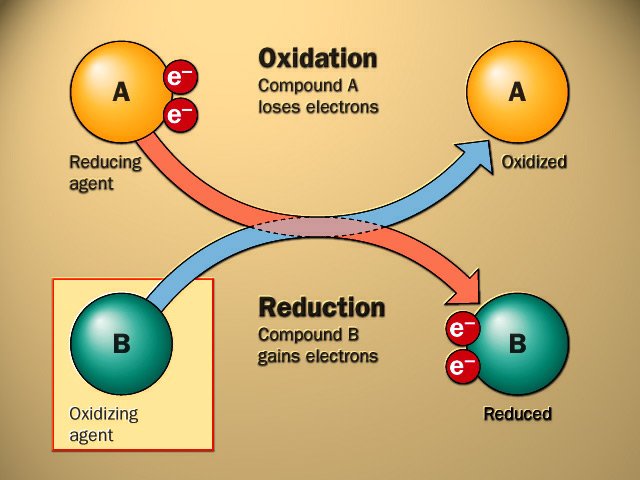
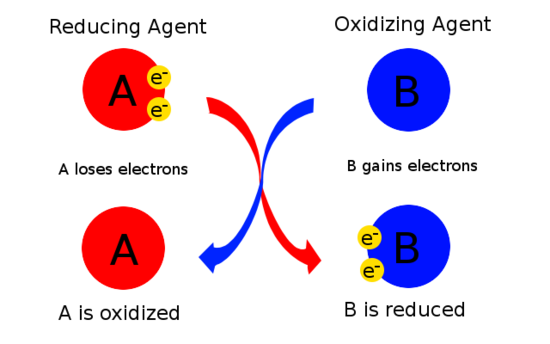
Oxidation And Reduction Occur Together
Once the electron was discovered and chemical reactions could be explained, scientists realized oxidation and reduction occur together, with one species losing electrons and another gaining electrons . A type of chemical reaction in which oxidation and reduction occurs is called a redox reaction, which stands for reduction-oxidation.
The oxidation of a metal by oxygen gas could then be explained as the metal atom losing electrons to form the cation with the oxygen molecule gaining electrons to form oxygen anions. In the case of magnesium, for example, the reaction could be rewritten as:
2 Mg + O2 2
comprised of the following half-reactions:
Mg Mg2++ 2 e-
O2+ 4 e- 2 O2-
Oxidation And Redox Potentials
For a half-reaction with a given oxidation potential, its reduction potential will be opposite in sign.The overall potential of a redox reaction is the sum of the reduction and oxidation half-reaction potentials.
- The potential of a chemical cell is a sum of the potentials of the half reactions.
- When adding the half-cell potentials, make sure that there is a reduction and an oxidation taking place.
- The positive electrode is called the cathode, and the negative electrode is called the anode.
You May Like: What Is Ucr In Psychology
Changes In Oxidation Number
Redox reactions can be recognized, as the change of the oxidation number of some of the atoms. If the oxidation number of an element increases, then the element is oxidized. If it is decreased, then the element is reduced.
- The oxidation number is in the range between -7 and +7. It shows the number of electrons lost with regards to the neutral atom.
- The reducing agent is an element or compound that can lose an electron .
- The oxidizing agent is an element or compound that can gain an electron .
Why Is Oxygen So Reactive
When oxygen reacts with any other element , electrons are pulled towards the oxygen and away from the other element in the bond. This attraction of the shared electrons by the oxygen results in the formation of a negative ion, which will react with any positively charged molecule.
Distribution of charges in a molecule of water. The slightly negative field around the oxygen results from its ability to pull electrons away from other elementsin this case, hydrogen. The hydrogens then have a slightly positive field.
Learn more about chemical bonding.
Powered by WordPress / Academica WordPress Theme by WPZOOM
Read Also: How To Avoid Parallax Error In Physics
How Do You Recognize Redox Reactions
The easiest and primary way to recognize a redox reaction is by look for changes in the oxidation states of atoms from the reactants to the products. One species should have its oxidation number decrease from reactants to products . Another species in the same reaction should have its oxidation number increased from reactants to products . Just remember the number of electrons lost should be equal to the number of electrons gained.
Reminder: an atoms oxidation number is a measure of how many electrons it has gained or lost. That is, an oxidation number of zero indicates a neutral atom. Similarly, gaining electrons decreases the oxidation number, since electrons are negative and thus add a negative charge. Losing electrons increases the oxidation number, since the negative charge is being lost. Read more about oxidation states here!
Putting The Ox In Redox
Oxidation and reduction always, always, occur together.
For purely theoretical approaches, half-reactions can be used to explain half of a redox reaction be it the oxidation or the reduction component. These are pretty helpful in simplifying the whole process, to make it easier to teach or understand. But keep in mind the first line: in real life, oxidation and reduction always come together.
Quite simply, an electron wont want to leave its hosting atom. It wont go into the wild willy-nilly. Theres nothing to satisfy its electrical imbalance there. But having a more inviting host nearby to move on to can draw it out. Oxidation, then, cannot occur unless theres an electron-thirsty atom around. On the other hand, without an electron donor, theres no transfer. Reduction, then, cant occur if theres nobody to strip electrons from.
Think of it as a marketplace. You need buyers to have sellers and vice-versa one simply cant happen without the other.
Ok, so why do we call it reduction? Again, its history at work. We werent able to properly understand chemistry for quite a long time, but we were able to observe and measure some of its effects. Reduction is actually a metallurgic term. Smelters could see that refining a one-pound piece of ore would net less than a pound of metal. They didnt know why, but they could see the drop in quantity, so they referring to it as reducing the ore to its base metal.
You May Like: How To Find Ksp Chemistry