The Selection Of Parent Chain:
The first step in naming an organic compound is to select the parent chain and give the root word based on the number of carbon atoms in it.
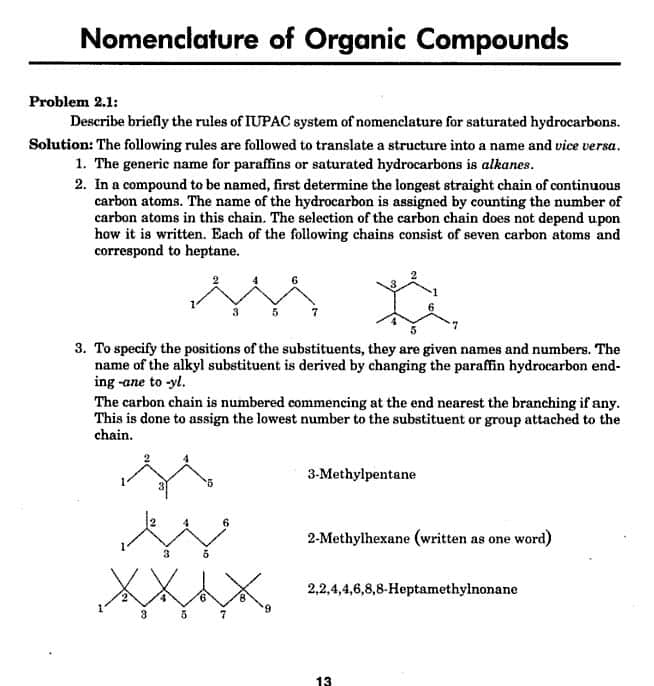
The parent chain in an organic molecule is the longest continuous carbon chain containing as many functional groups, double bonds, triple bonds, side chains and substituents as possible.
Examples:
i) In the following molecule, the longest chain has 6 carbons. Hence the word root is “hex-“. Note that the parent chain may not be straight.
ii) The root word for the following molecule is “hept-” since the longest chain contains 7 carbons.
Do not come under the impression that the ethyl groups are side chains and the longest chain contains 5 carbons.
The shaded part shows the longest chain that contains 7 carbons. Also look at the alternate way of writing this molecule in which the ethyl groups are expanded to -CH2CH3.
iii) In the following molecule, there are three chains of equal length .
However, the chain with more number of substituents is to be taken as the parent chain. Thus “hept” appears as word root in the IUPAC name of this compound.
iv) The double bonds and triple bonds have more priority than the alkyl side chains and some other substituents like halo, nitro, alkoxy etc. Hence, whenever there are two or more chains with equal number of carbons, the chain that contains double or triple bond is to be selected as the parent chain irrespective of other chain containing more number of substituents.
Number The Carbon Atoms
Remember that the carbon atoms must be numbered so that the functional group is at the lowest numbered carbon atom possible. In this case, it doesn’t matter whether we number the carbons from the left to right, or from the right to left. The double bond will still fall between the second and third carbon atoms.
How Do You Identify Organic Compounds
Organic compound, is one of a large class of chemical compounds in which one or more carbon atoms are covalently associated with other elements atoms, most commonly hydrogen, oxygen, or nitrogen. The few compounds that contain carbon that are not classified as organic include carbides, carbonates, and cyanides.
You May Like: What Math Courses Are Required For Psychology Major
Alphabetize Multiple Substituent Types
Panic usually sets in when multiple types of substituents occur on the same molecule.
Dont panic!
And certainly dont try to name the entire compound in one shot.
Instead, write out your puzzle pieces one at a time. In my Organic Chemistry IUPAC Naming videos you will notice that I mark off every component as I address it, by highlighting chains or circling substituents.
Lets apply this approach to a simple multi-substituted compound pictured here. Then follow the puzzle piece approach as follows:
Note on 2,4-dimethyl
Notice that there are two indications of the fact that there are two methyl groups present:
di indicates that there are 2 groups.
2,4 indicates the carbon atom where each methyl group occurs.
Now that we have a simple list of substituents lets put it all together. We have no functional group and so we follow the pattern of
prefix first name last name
But we have a problem. There are two sets of substituents present.
When faced with more than one type of substituent, order them alphabetically.
The di in dimethyl is an adjective and is not counted for alphabetical order.
Steps Involved In Writing Iupac Name
1) The first step in giving IUPAC name to an organic compound is to select the parent chain and assign a word root.
2) Next, the appropriate primary suffix must be added to the root word to indicate the saturation or unsaturation.
3) If the molecule contains functional group or groups, a secondary suffix must be added to indicate the main functional group. This is optional and not necessary if the molecule contains no functional group.
4) Prefix the root word with the infix “cyclo” if the parent chain is cyclic or with the infix “spiro” if it is a spiro compound or with the infix “bicyclo” if the compound is bicyclic.
5) Finally add prefix to the IUPAC name, if there are side chains or substituents on the parent chain.
E.g. The IUPAC name of the following compound is arrived in steps mentioned below.
Now add them to makeup the IUPAC name of the compound.
You will learn how to select a parent chain? how to number the carbon atoms and give the locants to the functional groups, side chains ? etc., in the following section.
Don’t Miss: What Is An Emulsion In Organic Chemistry
How To Name Organic Compounds
wikiHow is a wiki, similar to Wikipedia, which means that many of our articles are co-written by multiple authors. To create this article, 9 people, some anonymous, worked to edit and improve it over time. This article has been viewed 46,342 times.
This is the best and simplest guide to naming compounds with hydrogen and carbon in them! Don’t be put off by the long and complicated names, start from the basics.
Look For Any Branched Groups Name Them And Give Their Position On The Carbon Chain
There is a branched group attached to the second carbon atom. In this case the methyl group is on carbon 2 regardless of which side you number the longest chain from.
This group has the formula \, which is methane without a hydrogen atom. However, because it is not part of the main chain, it is given the suffix -yl . The position of the methyl group comes just before its name .
Recommended Reading: What Is Q2 In Math
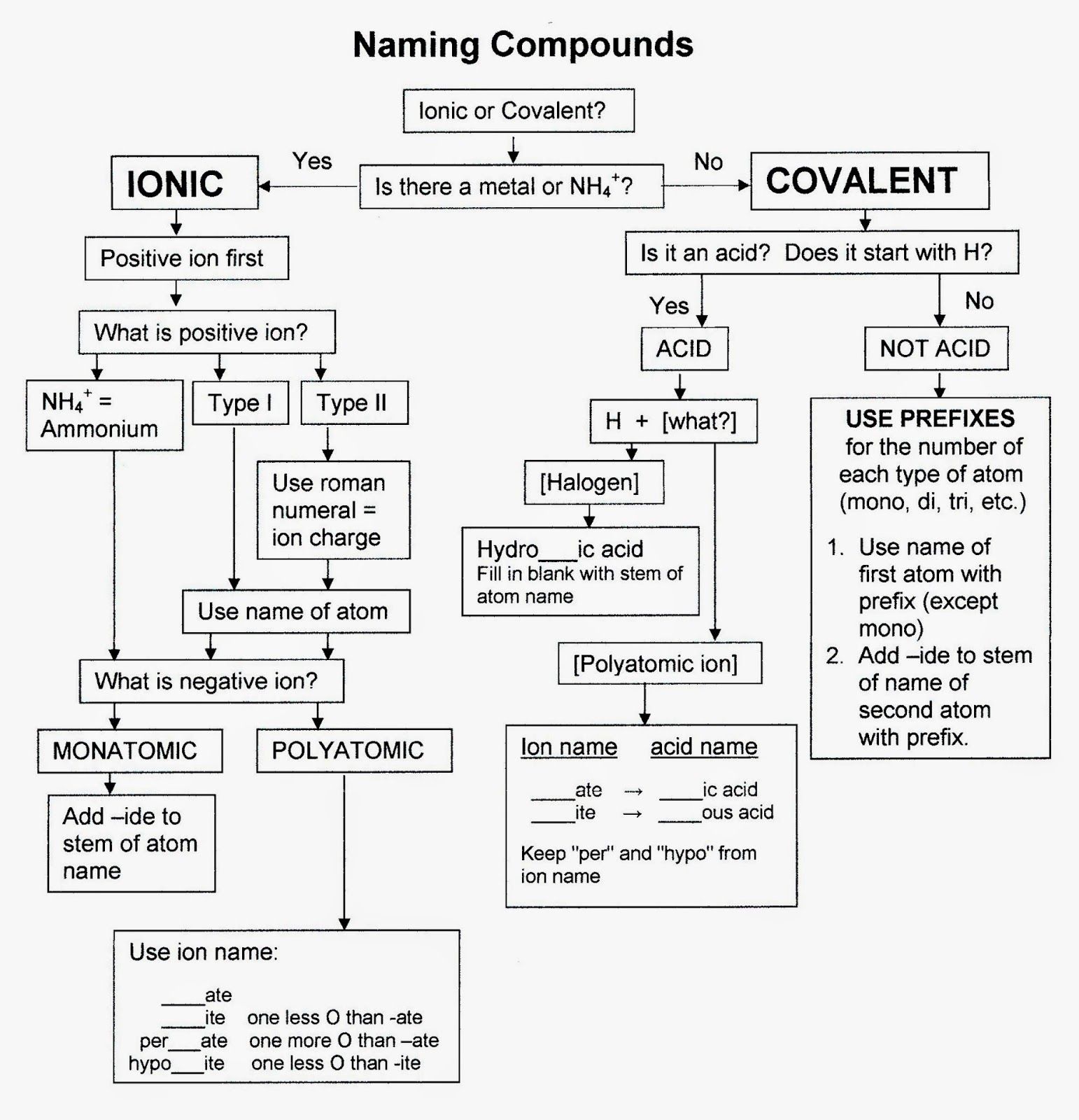
What Is The Nomenclature Of Organic Compounds
Nomenclature means the system of naming organic compounds based on specific guidelines. This is also quite necessary otherwise, it will be very difficult to assign names to the vast number of organic compounds and remember them. There are two systems of Nomenclature, namely:
A non-systematic name for a chemical compound is known as a trivial name in chemistry. That is, according to the rules of any formal system of chemical nomenclatures, such as IUPAC inorganic or IUPAC organic nomenclature, the term is not accepted.
2. IUPAC Nomenclature of Organic Compounds
Organic chemistry deals with a huge number of different molecules. A systematic way of naming has been devised to clearly identify them, and it is known as the IUPAC .
Putting It All Together: A Practice Example
But lets try a quick example. Here we have a 5-carbon chain with a CH3 functional group and CHO at the end.
We break this down as follows:
- 5 carbons in the parent chain for a first name of pent
- Only single bonds for a last name of ane
- Functional group on the right so we start numbering at the CHO
- CH3 on carbon-4 for a prefix of 4-methyl
- Aldehyde on the first carbon for a last name of al
Note that terminal functional groups such as carboxylic acid, aldehydes and more are implied to be on the first carbon and thus requires no numerical designation.
In putting the name together we follow the format of prefix -first name last name suffix
One final adjustment. Since al starts with a vowel and ane ends in a vowel, we drop the e in ane allowing the name to flow better for a final name of 4-methylpentanal
For even more organic chemistry IUPAC nomenclature tutorials, visit my website Leah4sci.com/naming for my complete 21 organic chemistry nomenclature video series taking you through the basics all the way to individual functional groups.
Thank you to Leah for writing this epic post about Organic Chemistry IUPAC Nomenclature! You can also follow Leah on Twitter at @Leah4Sci
Read Also: What Is Diffusion In Geography
Numbering The Parent Chain:
i) The positions of double bonds or triple bonds or substituents or side chains or functional groups on the parent chain are to be indicated by appropriate numbers . The locants are assigned to them by numbering carbon atoms in the parent chain.
Even though two different series of locants are possible by numbering the carbon chain from either sides, the correct series is chosen by following the rule of first point of difference as stated below.
Note: In iupac nomenclature, the number which indicates the position of the substituent is called ‘locant’.
The rule of first point of difference:
When series of locants containing the same number of terms are compared term by term, that series which contains the lowest number on the occasion of the first difference is preferred.
For example, in the following molecule, the numbering can be done from either side of the chain to get two sets of locants. However the 2,7,8 is chosen since it has lowest number i.e., 2 on the first occasion of difference when compared with the other set: 3,4,9.
Actually the so called Least Sum Rule is the special case of above Rule of First point of Difference. Though looking simple, the least sum rule is valid only to chains with two substituents, a special case. However use of Least sum rule is not advisable when there are more than two substituents since it may violate the actual rule of first point of difference.
But note that the ethyl group is written first in the name.
Organic Compounds Follow A Similar Naming Pattern
- Prefix = substituent
- First Name = carbon chain number
- Last Name = type of chain
- Suffix = highest priority functional group
And so your simple approach is as follows: When you come across a complex molecule with multiple components to name, identify each one individually. Put its name on a list then view the list items as a puzzle that must be put together in a logical sequence.
You May Like: Can Quantum Physics Explain Consciousness
Common Names In Organic Chemistry
The IUPAC nomenclature is the standardized official naming rule of organic compounds. Opposed to that, common names are older names for the compounds, which are not official, but some are frequently used. There is no explicit rule for common names, and they are used interchangeably with IUPAC names, so one must get acquainted to both naming conventions.
Contents
Video 1 Introduction To Iupac
The rules for naming organic compounds are tedious and can become overwhelming fast. The first video shows you how to break down the name of an organic molecule using my puzzle piece approach’. This video is a MUST for breaking down nomenclature in a simple and fun-to-solve manner.
Looking for how to name Cis/Trans or E/Z compounds? Read HERE!
Or, need even more naming practice? Watch the video below, and then continue on by trying my Naming Organic Compounds Practice Quiz .
You May Like: What Are Growth Factors In Biology
Parent Chain Length The Molecules First Name
Count the number of carbon atoms after identifying and highlighting your parent chain. I recommending actually numbering your molecule. This is a good habit to develop NOW as it will provide a reference point later on when you have to name multiple substituents and functional groups.
You will assign a first name to your molecule based on the number of carbons present in the parent chain as follows:
An alkane is a saturated molecule which does not contain any double bonds.
An alkane has a last name of ane
An Alkene is partially unsaturated and contains a least one double or pi bonds.
An alkene has a last name of ene
An Alkyne is the most unsaturated and contains a triple bond. This is 2 pi or double bonds between the same 2 carbon atoms.
An alkyne has the last name of yne
Iupac Name Of Compounds With Multi Functional Groups
Whenever there are more than one functions group, the main functional group is indicated by the 2osuffix in the IUPAC name, whereas the remaining functional groups are considered as substituents and are indicated by the appropriate prefixes.
E.g. In the following organic compound, 5-hydroxyhexanoic acid, both -OH and -COOH groups are the functional groups. But the -COOH group has more priority than the -OH group. Hence it is considered as the main functional group and indicated by secondary suffix, “oic acid”. Whereas the -OH group is considered as substituent and is indicated by the prefix, “hydroxy”.
Don’t Miss: How To Find Concentration In Chemistry
R Is The Rest Of The Molecule
When you see R anywhere on your molecule, recognize that this represents the Rest of the molecule. However, to keep things simple, and given that we are not looking at that portion of the molecule, just cut it all out and draw R in its place. For the purpose of branched substituents, R will represent the invisible parent chain.
Methyl and ethyl substituents are short substituents and can have no branched isomers.
A propyl substituent has a single isomer as pictured.
Propyl is a 3-carbon substituent. When connected in sequence we simply call it propyl, however, when connected to the parent chain by the second instead of the first carbon, it gets the name isopropyl.
Iso is a group that you will see again later so recognize that an iso-tail is like a mermaids tail or fork in the road.
A butyl substituent has four carbons in a row. With more carbons we get more isomer opportunities, in fact, butyl has a total of four isomers as follows:
- Butyl has all four carbons in a row, attached to the parent by the first carbon.
- Secbutyl or 1-methylpropyl all four carbons still in a row, but secbutyl is attached to the parent by the second or secondary carbon
- Isobutyl or 2-methylpropyl has a forked or iso-tail on a 3-carbon substituent chain.
- Tertbutyl or 1,1-dimethylethyl is unique in that it has 2 methyl branches coming off the first carbon in a 2-carbon chain.
Example Of Iupac Nomenclature
Considering the following Example:
- There exist 9 carbon atoms on the straight chain and the 5th carbon atom consists of a substituent group which in turn has 3 carbon atoms in a chain.
- Furthermore, there the first and second carbons of this substituent chain have an additional CH group attached to them.
- In the nomenclature of this compound, the 9 membered carbon chain is identified as the parent chain and is numbered.
- The substituent chain attached to position 5 of the parent chain is 3 members long, with 2 methyl groups attached at positions 1 and 2.
- Thus the carbon chain substituent group on the parent chain can be called 1,2 dimethyl propane. The name for the substituent chain containing this compound would be 1,2 dimethyl propyl.
- Substituting this name on the parent chain, the IUPAC name of the compound in question is found to be: 5- nonane.
Don’t Miss: Pre Algebra 6th Edition Answers
Lets Name Those Organic Suckers
Do you get overwhelmed when it comes to naming organic compounds? Or do you get excited and accept the challenge of naming them?
When I was young, I used to dread naming them. Couldnt be bothered spending time learning the rules . It was much easier to brush it off claiming its not important and will not come in handy. Well that worked for a while until I chose Chemistry as my major in undergraduate and later turned it into my career! Ha! Time to get serious and learn to name those suckers. Many organic compounds later Its actually very exciting once you figure out the basics.
I do empathize with anyone who is facing the same lack of love for naming compounds using IUPAC name as I used to be in the same shoes. Would you believe me if I say you can pick up the basics of naming all the 13 functional groups in less than 20 minutes? To be clear, I am referring to naming mostly simple organic compounds here. After all, we need to first build a solid foundation. Baby steps. Get your pen and paper ready!
Ready to test your skills? Here are 3 practice sets. Note: The structures are drawn in skeletal form. Check out here or here if you need a quick refresher.
Nomenclature Of Organic Compounds: Rules Examples
Nomenclature means the system of naming organic compounds based on specific guidelines. A set of logical rules was devised and used by organic chemists to circumvent problems caused by arbitrary nomenclature. Nomenclature of organic compounds is defined as the systematic approach taken for the nomenclature of the organic compounds as per the recommendation of IUPAC.
The name of an organic compound involves three parts namely, a word root, suffix, and prefix. In this article, we will discuss about the nomenclature of organic compounds in detail. Scroll down to learn more!
You May Like: What Is Aerobic Respiration In Biology
Nomenclature Of Organic Compounds Examples
We know that the IUPAC name of a compound consists of word root, primary and secondary suffixes. While writing the complete IUPAC name of a compound following points are necessary to be followed
The sum up IUPAC name of an organic compound consists of the following arrangement.
Some of the examples are given below:
1. \
This compound contains one carbon atom hence word root is Meth- and it contains single bond, so the primary suffix is -ane.
Thus, the IUPAC name of this compound is Methane.
Prefix: methyl
Primary suffix: -ane
Secondary suffix: -ol
Thus, the complete IUPAC name of the compound can be written as
\} }\mathop }}\limits_}\,}} \right)} }\mathop }}\limits_}}\,}} \right)} }\mathop }\limits_}}\,}} \right)} }\)
Hence, the IUPAC name of given compound is \-methylpropan\-ol
Ans: The compound is substituted octane It has branches at carbon -\, carbon-\ and carbon-\. Thus, the name is \-sec-butyl-\-ethyl-\-methyloctane.