Effects Of Heat Treatment
Milk is heat treated at the dairy to kill any pathogenic microorganisms that may be present. Heat treatment also causes changes in the constituents of the milk. The higher the temperature and the longer the exposure to heat, the greater the changes. Within certain limits, time and temperature can be balanced against each other. Brief heating to a high temperature can have the same effect as longer exposure to a lower temperature. Both time and temperature must therefore always be considered in connection with heat treatment.
Fat
During denaturation -casein adheres to -lactoglobulin.
Protein
Enzymes
Enzymes can be inactivated by heating. The temperature of inactivation varies according to the type of enzyme. There are some bacteria, Pseudomonas spp, , which are very often cited among the spoilage flora of both raw cold-stored milk and heat-treated milk products they have extremely heat-resistant proteolytic and lipolytic enzymes. Only a fraction of their activity is inhibited by pasteurization or UHT treatment of the milk.
Lactose
Vitamins
The Interface Cannot Be Seen
On occasion the compounds in a separatory funnel are so dark that they obscure the interface between the two layers. If this happens, there are several methods that might help you see the interface. One is to hold the separatory funnel up to the light, or to shine a flashlight onto the glass . Additional light sometimes allows you to see the interface. A second method is to carefully observe the layers while tilting the funnel back and forth to the side . Your eye can sometimes pick up on subtle differences in the way the liquids flow. A third method is to add a bit more solvent to the funnel to somewhat dilute one of the layers, or to add a different solvent to alter the index of refraction.
Applications And Uses Of Emulsion
Emulsions are very much famous in various fields of science. It is utilized in the tanning and dyeing industries, used in the manufacturing process of plastics and synthetic rubber.
- Usually used in cosmetics, pharmaceuticals, personal hygiene.
- Microemulsions are used to deliver vaccines to kill various microbes.
- It is used in chemical synthesis mainly in the manufacture of polymer dispersions.
- It is used in firefighting.
- Nanoemulsions such as soybean oil are used to kill microbes.
- Mayonnaise is an oil in water emulsion with egg yolk or sodium stearoyl lactylate.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
Don’t Miss: Punchline Bridge To Algebra 2nd Ed
Double Emulsions As Templates For Vesicles
Water/Oil/Water emulsions stabilized with phospholipid surfactants can be formed using the typical capillary methods for creating multiple emulsions in microfluidics. If a volatile organic compound is chosen as the oil phase for the emulsion, the phospholipids adsorbed to the two surfaces are brought into contact as the oil phase is a evaporated, and thus, a vesicle is formed. Vesicles are a promising application of double emulsions which may enable applications including drug delivery.
Emulsions Are Created By Surfactants
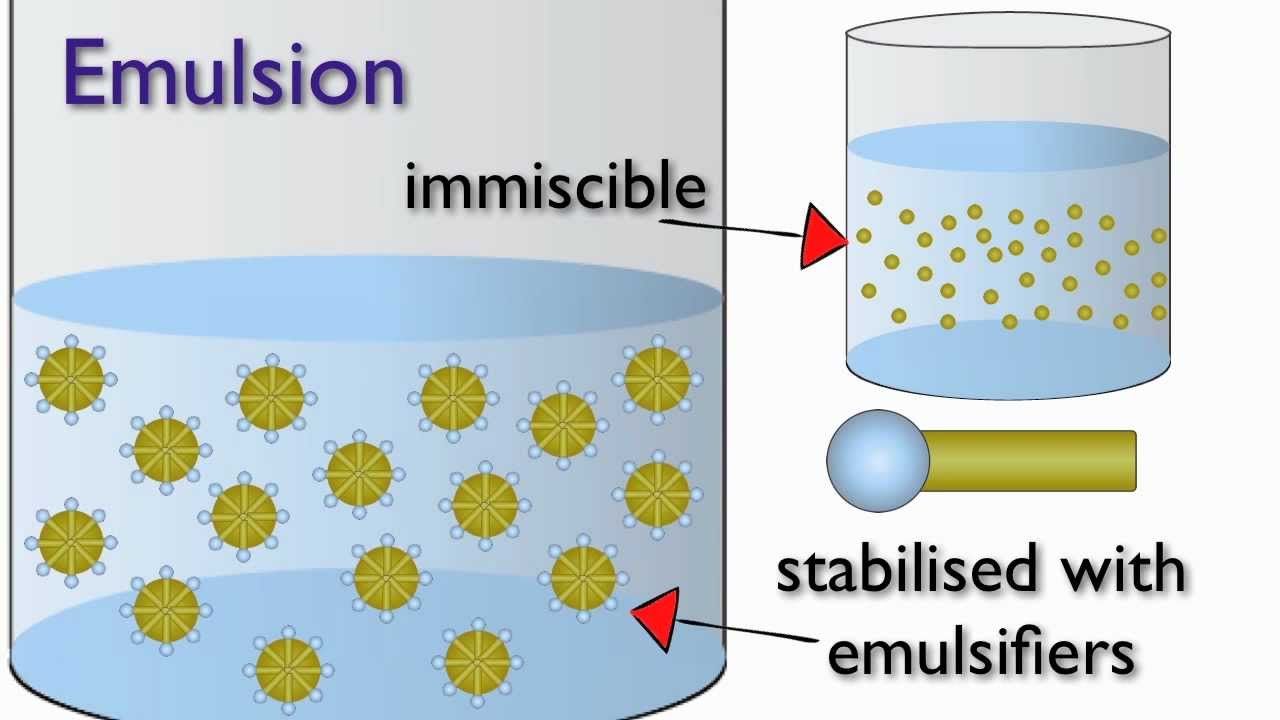
An emulsion can be described as a collection of tiny droplets of one liquid dispersed in another liquid in which it, the first liquid, is insoluble. Emulsions are formed by mixing these two liquids with a third substance, known as an emulsifier, which creates a uniform, stable dispersion of the first liquid in the second.
Emulsifiers belong to the class of chemicals known as surfactants, or surface active agents, which are able to reduce the surface tension of liquids. This ability is important because surface tension, must be overcome for the two liquids to effectively intermingle. Surfactants are able to create this effect by virtue of the dual nature of their molecular structure. One part of the molecule is soluble in water, the other in oil therefore, when an emulsifying surfactant is added to a mixture of oil and water it acts similar to a bridge between the two immiscible materials. This bridging effect reduces the forces between the liquid molecules and allows them to be broken into, and maintained as, separate microscopic droplets.
Read Also: Figure And Ground Psychology
The Layers Don’t Separate Well
Emulsions are when tiny droplets of one layer are suspended in the other layer, resulting in no distinct interface between the two layers . Often an emulsion looks like a bubbly mess near the interface, and can even appear to be an odd-looking third layer.
Emulsions can happen for several reasons:
Emulsions can be very difficult to rectify, and it’s best if they are avoided in the first place by shaking solutions that are prone to emulsions gently in the separatory funnel. Nonetheless, if an emulsion does form, there are some ways to attempt to clarify them:
Setting Your Browser To Accept Cookies
There are many reasons why a cookie could not be set correctly. Below are the most common reasons:
- You have cookies disabled in your browser. You need to reset your browser to accept cookies or to ask you if you want to accept cookies.
- Your browser asks you whether you want to accept cookies and you declined. To accept cookies from this site, use the Back button and accept the cookie.
- Your browser does not support cookies. Try a different browser if you suspect this.
- The date on your computer is in the past. If your computer’s clock shows a date before 1 Jan 1970, the browser will automatically forget the cookie. To fix this, set the correct time and date on your computer.
- You have installed an application that monitors or blocks cookies from being set. You must disable the application while logging in or check with your system administrator.
Don’t Miss: Formula For Volume Physics
Formation Of Emulsion And Its Type
Emulsion is a dispersed system in which the phases include immiscible or partially miscible liquids, such as milk and rubber latex. If an emulsion is prepared by homogenizing two pure liquid components, phase separation will generally be rapid, especially if the concentration of the dispersed phase is at all high. To prepare reasonably stable emulsions a third component, an emulsifying agent , must be present, such as surfactant or solid powder. The functions of the emulsifying agent are to facilitate emulsification and promote emulsion stability. The stabilizing mechanism is generally complex and may vary from system to system however, two of them are very common and very important.
- 1.
-
Low interfacial tension: The adsorption of surfactants at the interface of phases causes a lowering of interfacial energy, thus facilitating the development of emulsion and enhancing the stability of the large interfacial area associated with emulsion.
- 2.
-
A mechanically strong interfacial film: The stability of emulsion arises from the mechanical protection given by the adsorbed films around the droplets, rather than from a reduction of interfacial tension.
In nearly all emulsions, one of the phases is aqueous and the other is oil . Thus the emulsions are grouped into two types according to the properties of phases as follows.
There are three methods by which the emulsion type may be identified:
Vikas Jhawat, … Anil Kumar Sharma, in, 2021
Basic Physical Chemical Properties Of Cows Milk
Cows milk consists of about 87 % water and 13 % dry substance, table 2.1. The dry substance is suspended or dissolved in the water. Depending on the type of solids and size of particle , there are different distribution systems of them in the water phase.
Physical-chemical status of cows milk.
| Average composition % |
|---|
Relative sizes of particles in milk.
| Size |
|---|
| Lactose, salts and other substances in true solutions |
Recommended Reading: Molecular Shape For Ccl4
Bancroft’s Rule And The Hlb
Bancroft’s Rule:
| The emulsifier stabilizes the emulsion type where the continuous phase is the medium in which it is most soluble. | A hydrophilic solute in an O/W emulsion. |
|---|---|
| The long tail on the surfactant is to represent the longer range interaction of a hydrophilic molecule through water. | A hydrophilic solute in a W/O emulsion. |
The Hydrophile-Lipophile Schema:
Organic Layer Is On The Bottom
In this section are stepwise instructions on how to extract an aqueous solution with an organic solvent that is denser than water . As an example, the instructions are written to extract an aqueous solution three times using \ each time , Figure 4.30).
Extraction #1
Extraction #2
Extraction #3
Read Also: Half Life Equations Chemistry
Texturas By Ferran Adria
In his recent visit to Harvard, world-famous chef and culinary innovator Ferran Adria gave several video demonstrations of his cutting-edge cooking techniques. He spoke of his desire to create a new language of food. Some chef in the past made the first salad, the first soup, and the first bread, and now there are thousands of varieties of each. Each of these examples could be considered new “words” that could create a new world of culinary possibilities. Over the past decades, he has been searching for fundamental advances in cooking that could be considered new letters of the culinary alphabet.
This new alphabet is exemplified by Albert and Ferran Adria’s new line of Texturas, various chemicals that enable new culinary experiences. Below are some specific cases related to emulsions, including annotated recipes:
Lecite:
Frozen Parmesan air
- 450g water
- 3g Lecite’
Sucro:
Olive oil spiral: For virgin olive oil caramel
- 100 g of Isomalt
- 45 g of extra virgin olive oil
- 1.5 g of Glice
Glice:
Black olive emulsion
Difference Between Emulsion And Colloid
Sometimes the words emulsion and colloid are used interchangeably, but they dont mean quite the same thing. An emulsion is a type of colloid. A colloid, in turn, is a type of homogeneous mixture. All emulsions are colloids, but not all colloids are emulsions. An emulsion is a colloid in which all the phases are liquids. There are other types of colloids, defined according to their phases. For example, an aerosol is a solid dispersed in a gas , while a foam is as gas dispersed in a liquid .
You May Like: What Does Abiotic Mean In Biology
What Is Polymer Emulsion
Polymer emulsion means water-soluble polymers, such as certain polyvinyl alcohols or hydroxylate celluloses, can also be used to act as emulsifiers/stabilizers.
Explanation:
Emulsion polymerization is a type of radical polymerization that usually starts with an emulsion incorporating water, monomer, and surfactant. The most common type of emulsion polymerization is an oil-in-water emulsion, in which droplets of monomer are emulsified in a continuous phase of water.
Emulsion polymerization is used to manufacture several commercially important polymers. Many of these polymers are used as solid materials and must be isolated from the aqueous dispersion after polymerization.
Advantages of emulsion polymerization include
High molecular weight polymers can be made at fast polymerization rates. By contrast, in bulk and solution free radical polymerization, there is a trade off between molecular weight and polymerization rate.
The continuous water phase is an excellent conductor of heat, enabling fast polymerization rates without loss of temperature control.
Disadvantages of emulsion polymerization include:
1.Surfactants and other polymerization adjutants remain in the polymer or are difficult to remove.
2.For dry polymers, water removal is an energy-intensive process.
Organic Layer Is On The Top
In this section are stepwise instructions on how to extract an aqueous solution with an organic solvent that is less dense than water . As an example, the instructions are written to extract an aqueous solution three times using \ diethyl ether each time diethyl ether). A procedural summary of the first two extractions is in Figure 4.29.
Extraction #1
Extraction #2
Extraction #3
Don’t Miss: Mcdougal Littell Geometry Book Answers
Accelerating Methods For Shelf Life Prediction
The kinetic process of destabilization can be rather long â up to several months, or even years for some products. Often the formulator must accelerate this process in order to test products in a reasonable time during product design. Thermal methods are the most commonly used â these consist of increasing the emulsion temperature to accelerate destabilization . Temperature affects not only the viscosity but also the interfacial tension in the case of non-ionic surfactants or, on a broader scope, interactions between droplets within the system. Storing an emulsion at high temperatures enables the simulation of realistic conditions for a product , but also accelerates destabilization processes up to 200 times.
Mechanical methods of acceleration, including vibration, centrifugation, and agitation, can also be used.
These methods are almost always empirical, without a sound scientific basis.
Emulsions: Making Oil And Water Mix
After reading this article, you will understand:
- the basic science of emulsions
- how formulators choose which emulsifier to use for a particular emulsion
- how emulsifiers are used in foods, nutraceuticals, personal and home care products, industrial lubricants, environmental technologies, biofuels, and other applications.
The immiscibility of oil and water has inspired the proverb Oil and water dont mix and other expressions that reflect the general incompatibility of two entities, such as My coworker and I are like oil and water. Yet within our homes are numerous examples of products in which oil and water do mix: mayonnaise, milk, salad dressings, hand lotion, and hair conditioner, to name but a few. These examples represent emulsions, which are stable mixtures of tiny droplets of one immiscible fluid within another, made possible by chemicals called emulsifiers.
How emulsions and emulsifiers work
Simple emulsions are either oil suspended in an aqueous phase , or water suspended in oil . Milk is an example of an o/w emulsion, in which the fat phase or cream forms tiny droplets within the skim milk, or water phase. In contrast, margarine is a w/o emulsion containing droplets of water or skim milk in a blend of vegetable oils and fat. In both cases, emulsifiers are needed to prevent the suspended droplets from coalescing and breaking the emulsion.
How to choose the right emulsifier
Macro- and microemulsions
Foods
Nutraceuticals
Personal care
Home care
Sidebar
Recommended Reading: Physics Volume Formula
Minerals And Salts In Milk
Milk contains a number of minerals. The total concentration is less than 1 %. Mineral salts occur in solution in milk serum or in casein compounds. The most important salts are those of calcium, sodium, potassium and magnesium. They occur as phosphates, chlorides, citrates and caseinates. Potassium and calcium salts are the most abundant in normal milk. The amounts of salts present are not constant. Towards the end of lactation, and even more so in the case of udder disease, the sodium chloride content increases and gives the milk a salty taste, while the amounts of other salts are correspondingly reduced
Other constituents of milk
Milk always contains somatic cells . The content is low in milk from a healthy udder, but increases if the udder is diseased, usually in proportion to the severity of the disease. The somatic cell content of milk from healthy animals is as a rule lower than 200 000 cells/ml, but counts of up to 400 000 cells/ml can be accepted. Milk also contains gases, some 5 6% by volume in milk fresh from the udder, but on arrival at the dairy, the gas content may be as high as 10% by volume. The gases consist mostly of carbon dioxide, nitrogen and oxygen.
They exist in the milk in three states:
Dispersed and dissolved gases are a serious problem in the processing of milk, which is liable to burn on to heating surfaces if it contains too much gas.