What Is Quench In Organic Chemistry
4.9/5Quenchingquenching
Subsequently, one may also ask, what is the quenching process?
In materials science, quenching is the rapid cooling of a workpiece in water, oil or air to obtain certain material properties. A type of heat treating, quenching prevents undesired low-temperature processes, such as phase transformations, from occurring.
One may also ask, why do we quench? Quenching is a rapid way of bringing metal back to room temperature after heat treatment to prevent the cooling process from dramatically changing the metal’s microstructure. Metalworkers do this by placing the hot metal into a liquid or sometimes forced air.
Beside above, how do you quench a Grignard reaction?
The classic way to quench a Grignard is to pour the ethereal solution DIRECTLY onto dry ice. The dry ice temperature moderates the vigour of the reaction. And of course this operation extends the carbon chain by ONE.
What is an acid work up?
An acid work–up is where acid, usually a mineral acid or perhaps acetic acid depending really upon how acid stable your compound is, is used to remove basic components of a reaction mixture, or to re-protonate a basic compound.
Dissociation Of Mgo And Mg2 Nanoparticles By Immersing In Respective Solutions
The sterilized solutions with respective MgO or Mg2 nanoparticles at the concentrations of 200 g/mL, 1000 g/mL, 2000 g/mL, and 3000 g/mL 2 only) were thoroughly suspended through pipetting and placed into the wells of sterile 12-well polystyrene culture plates in a biosafety-II laminar flow hood to maintain sterility and keep consistency with standard cell culture practices. The respective solutions of 3 mL without nanoparticles were included as the control group, referred to as the 0 g/mL group. The 12-well polystyrene culture plates were then placed in an incubator and incubated under standard cell culture conditions, i.e., 37 °C and 5% CO2, for 24 h to allow nanoparticles to dissociate in the conditions that closely mimic a physiological environment. After 24 h of incubation, the plates were removed from the incubator. The respective solutions with remaining particles were collected from each well and transferred into 15-mL conical tubes. Each tube was then centrifuged at 3000 rpm for 2 min to collect the particles as a pellet the supernatant from each tube was then transferred into a new 15-mL conical tube without disturbing the pellet. To rinse the particles, the pellet was suspended in 100% pure ethanol and centrifuged at 3000 rpm for 1 min. The ethanol was then aspirated out and new ethanol was added for storing the particles for further analyses of microstructure and composition. MgO and Mg 2 are both insoluble in ethanol .
Different Types Of Chemical Formulas
The chemical formula of a compound usually refers to its’ molecular formula, which represents the total number of atoms of each component element in 1 molecule of the compound. The chemical compounds’ compositions can be expressed in a variety of ways.
A Chemical Formula can be expressed in a variety of ways, including:
-
Empirical Formula- The ratio of the elements contained in a given chemical compound is represented by the empirical formula. The majority of empirical equations are derived through the study of experimental data. The molecular formulas can be used to derive empirical formulae.
-
Molecular Formula-The number of components in a given molecule may be determined using the molecular formula. The elements are represented by their symbols in molecular formulas, and the number of atoms in each given element in the molecule is indicated with the subscript.
-
Structural Formula- The structural formula of a given chemical compound supplies understanding into the configuration of the atoms in the given molecule.
Recommended Reading: Glencoe Geometry Workbook Answers
Analyses Of Respective Solutions After Immersion With Mgo And Mg2
Measuring pH of each solution
The pH of each immersion solution was measured immediately after separation from the particles, to minimize the changes due to temperature and atmospheric CO2, using a pre-calibrated pH meter .
Measuring Mg2+ ion concentrations using ICP-OES
Mg2+ ion concentration in each solution was measured after immersion of nanoparticles using inductively coupled plasma-optical emission spectrometry . Each solution collected after immersion was diluted to 1:200 in DI water. The solutions without nanoparticles were used to establish the baseline concentrations. This baseline concentration was subtracted from the measured Mg2+ ion concentrations for the groups with 2002000 g/mL 2) nanoparticles, and the reported values are the concentrations of Mg2+ released from MgO or Mg2 during immersion in respective solutions.
Morphological Changes In Mgo And Mg2 Nanoparticles After Immersion In Respective Fluids
Fig. 1
SEM images of MgO and Mg2 nanoparticles before and after immersion in respective physiologically relevant solutions for 24 h. SEM images with the scale bar of 2 m were all taken at an original magnification of ×10,000. For the SEM images in the insets and that have the scale bar of 200 nm, the original magnification was ×150,000
Recommended Reading: Accepted Value In Chemistry
Obtaining Of Magnesium Oxide:
Magnesium oxide obtained by calcination of minerals magnesite and dolomite.
It is the result of a chemical reaction the thermal decomposition of calcium carbonate and magnesium carbonate:
CaMg2 Cao + MgO + CO2
CaCO3·MgCO3 Cao + MgO + CO2
MgCO3 MgO + CO2
CaCO3 Cao + CO2 .
CaMg2, CaCO3·MgCO3 chemical formula of dolomite.
MgCO3 chemical formula of magnesite.
It is an industrial method for producing magnesium oxide.
Which Is Heat Taken By Mg To React With O 2
is heat taken by Mg to react with O 2 . Actually the oxidation/combustion reaction for MgO is an endo/exo thermic reaction. Its endothermic with the reactants.The total first and second ionization enthalpies for Magnesiums 3s 2 electrons are H = And exothermic for the products. I have a rusty nail here. Obviously iron reacted with oxygen.
Don’t Miss: What Does Thf Stand For In Organic Chemistry
Pt Clusters On Magnesium Oxide
Magnesium oxide is an ionic compound widely used as a substrate for diamond film, high-dielectric-strength film, and high-temperature superconducting film growth, finding additional medical, household, and industrial applications.
Hydrogenation of ethylene to ethane and the parallel hydrogenationdehydrogenation reaction producing ethylidyne are examples of transformations catalyzed by MgO-supported Pt clusters, which are categorized as structure-sensitive or structure-insensitive.
Size-selected Ptn clusters on MgO exhibit structure-sensitive ethylene hydrogenation activity at n 10, with maximum room-temperature activity observed for Pt13. In contrast, Pt clusters larger than 1 nm in diameter exhibit structure-insensitive activity ,1 revealing that the activity and/or selectivity of hydrogenation can be controlled and tuned by precise variation of catalyst particle size.
Nat. Commun.20167
Pt clusters also exhibit size-dependent CO oxidation activity with that of size-selected Ptn clusters on MgO surfaces shown in Fig. 2A and B .2 Whereas Ptn produced less than one CO2 molecule per cluster , the reactivity abruptly increased from Pt8 up to Pt15 with six CO2 molecules per cluster. The number of produced CO2 molecules per Pt atom shows a maximum of 0.4 for Pt15 and a minimum of 0.1 for Pt8. This is the first report of size-dependent reactivity of Pt clusters deposited on a metal oxide surface.
J. Am. Chem. Soc.1999121
How Is Magnesium Oxide Produced
Magnesium oxide is produced by the calcination of magnesium carbonate or magnesium hydroxide. The latter is obtained by treating solutions of magnesium chloride, sea water with lime water or milk of lime.
Mg2 + + Ca 2 Mg 2 + Ca2 +
Calcification at different temperatures produces magnesium oxide with different reactivity. High temperatures between 1500-2000°C reduce the available surface area and produce fully burnt magnesia it is a non-reactive form used as a refractory. Calcining temperatures of 1000-1500°C produce hard burnt magnesia with limited reactivity and low temperature calcination , producing lightly burnt magnesia, which is a reactive form and is also known as caustic calcined magnesia. Although some decomposition of carbonate into oxide occurs at temperatures below 700°C, the resulting materials seem to reabsorb carbon dioxide from the air.
Read Also: Algebra And Trigonometry 3rd Edition Stewart Pdf Free
Why Is Mgo Not A Covalent Compound
A covalent bond is formed when sharing of electrons is done between the atoms to achieve stability.
Usually, covalent bonds formed between the two nonmetals, between p-block and p-block, and formed when electronegativity difference between atoms exist less than 2.
Examples of some compounds that form covalent bonds H2O, NH3, H2S, SO2, NO2, AlCl3, etc.
Also Read:
According to the Pauling scale of electronegativity-
- The covalent bond is formed between the two atoms when their electronegativity difference occurs less than 1.7.
- The ionic bond is formed between the two atoms when their electronegativity difference occurs more than 1.7.
Now check the electronegativity difference in MgO compound-
The electronegativity value for magnesium atom = 1.31
The electronegativity value for oxygen atom = 3.44
The difference of the electronegativity between magnesium and oxygen atoms = 2.13
So, the difference in electronegativity between magnesium and oxygen is more than 1.7, hence, it is large enough to make an ionic bond between them as per the Pauling scale of electronegativity.
Also, in the MgO compound, there is no sharing of electrons involves, magnesium metal atom transfer its electrons and oxygen as nonmetal accept the electron to achieve stability by completing the octet.
And a bond is formed between the ions which is called an ionic bond.
The Kfactor Standard Ensures You Are Getting The Following:
raw and unpausterized honey
free of antibiotics, glyphosate and pesticides
NON-GMO
traceability from hive to home
comes from New Zealand
On top of that, there are two main varieties of Wedderspoons Manuka honey KFactor 16 and KFactor 12.
KFactor 16 is a monofloral honey that is wholly or mostly made from the Leptospermum scoparium plant.
This means this honey is more of a single-plant extract.
KFactor 12 is a multifloral Manuka honey, that is more of a blend of Manuka nectars and other floral types.
Recommended Reading: Error Of Parallax
Which Has A More Covalent Character Between Mgo And Cao
First of all, both MgO and CaO are ionic in nature because of the existence of a large electronegativity difference in value between the atoms of these compounds.
But no compound in the universe exists as 100% ionic. Therefore, MgO and CaO have some covalent characters even they are ionic in nature.
So, which has a more covalent character, MgO or CaO? MgO has a more covalent character than CaO, this is because Mg2+ has greater polarizing power than Ca2+, Hence, Mg2+ drawback the bonding electrons from O2- to a greater extent than Ca2+ corresponding to its high covalent character.
The Health Benefits Of Methylglyoxal
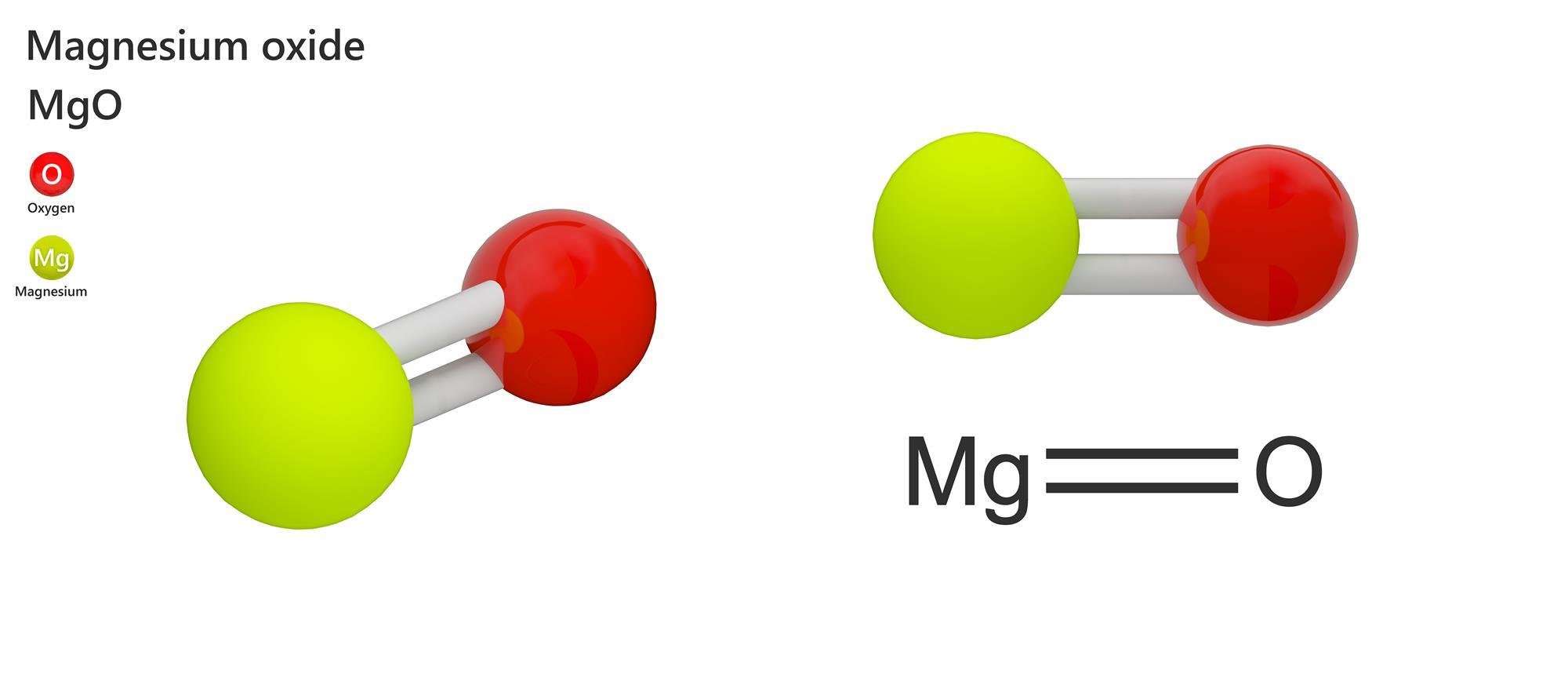
The MGO rating on a side of jar Manuka honey shows the methylglyoxal content. That means a MGO 100+ Manuka honey will have at least 100mg of methylglyoxal per kilogram.
What separates Manuka honey from regular honey is that the MGO levels can go up to 100 times. This is the reason many call it superfood.
Research that the most potent variety of Manuka honey coming from the manuka flower can have antibiotic properties. Talk about super bees!
Recently there have been successful studies on the efficacy of Manuka honey against skin bacteria and viruses. This can greatly help with acne.
It is also a natural antioxidant with a very powerful effect on wrinkles which makes it anti-aging solution.
As natural honey is known to be extremely nourishing it is also a great moisturiser. Hence many like to use this golden nectar as a face mask or face cream.
The higher the MGO content, the higher the antibacterial activity. This makes Manuka honey perfect for soothing conditions such eczema or psoriasis.
You May Like: Physics Vs Chemistry Which Is Harder
Unsoundness Of Portland Cement Containing Mgo And Cao
Dead burnt MgO and CaO if present in portland cement in the free form can promote volume expansion. A study was undertaken to evaluate the potentiality of thin disks as a replacement for the 25 by 25 by 282 mm prisms normally recommended in standard specifications for the autoclave test and also to compare the relative expansive nature of CaO and MgO.
A normal portland cement containing 1.28% MgO and 0.48% CaO was mixed with dead burnt MgO or CaO and made into disks 12.5 mm in diameter by a compaction load of 27.8 MPa or 31.25 mm in diameter at a load of 8.25 MPa . These disks, and the prisms made according to the ASTM Test for Autoclave Expansion of Portland Cement , were subjected to autoclave treatment at 2 MPa .
Figure 20 shows the relative autoclave expansion values for portland cement disks 12.5 mm pressed at 27.8 MPa containing MgO or CaO.
Figure 20. Autoclave expansion of portland cement compacts containing MgO or CaO.
Figure 21 compares the autoclave expansion of disks 31.25 mm in diameter pressed at 8.25 MPa with that of prisms containing different amounts of MgO. This work is also relevant to that discussed in the chapter on dimensional changes.
Figure 21. Comparison of autoclave expansion of portland cement compacts and prisms containing different amounts of MgO.
Anil Kumar K, … Suresh Gupta, in, 2021
How Are Metals In The Same Group React With Oxygen
As we have said, the metals in the same group will react in the same way as each other with oxygen. So, calcium reacts with oxygen in the same way as magnesium reacts with oxygen. The chemical equations also show similarities. The chemical equation for the reaction between calcium and oxygen is: 2Ca + O 2 2CaO
You May Like: Math Magic Tricks With Algebra
Is Mgo Ionic Or Covalent
Magnesium oxide, MgO, is considered an ionic compound. It contains two atoms, Magnesium which is metal, and Oxygen which is a nonmetal, both these are linked together by ionic bonding.
Also, the bond formed between magnesium and oxygen is ionic due to the large gap of electronegativity difference that exists between them.
| Name of Molecule |
Which Has More Covalent Character Mgo Or Beo
Both beryllium and magnesium belong to that same group and have the same valency with the same charge. But beryllium is present just above the magnesium.
As we go down the periodic group, the atom size increases. Hence, the magnesium is larger than beryllium.
Going by the trick to finding more covalent characters, use the C/S ratio formula.
C = Charge of cation
S = Size of the atom
Larger is the C/S ratio, more will be the covalent character.
So, both beryllium and magnesium have the same charge but the atomic size of magnesium is larger than beryllium.
Therefore, the C/S ratio of BeO is larger than the C/S ratio of magnesium.
The BeO has a more covalent character than MgO.
Recommended Reading: Are Michael Jacksons Kids His Biological Kids
Preparation Of Physiologically Relevant Solutions
Preparation of non-supplemented and complete DMEM
Dulbeccos modified Eagles Medium is a common medium for in vitro culture of adherent cells and was purchased from Fisher Scientific. It is common to supplement DMEM with 10% fetal bovine serum and 1% penicillin/streptomycin to obtain complete DMEM . Both DMEM without supplements and cDMEM with supplemental proteins from FBS and P/S were included for the immersion study to understand the effects of DMEM versus cDMEM. Non-supplemented DMEM is referred to as DMEM. All solutions were prepared in a laminar flow hood to maintain sterility.
Preparation of SBF
Preparation of HEPES
HEPES is a zwitterionic buffering agent that can maintain a biological pH between 6.8 and 8.2 and is not dependent on CO2 concentration in the incubator or air. A HEPES solution in DI water was made to match the same concentration of HEPES found in SBF. Specifically, 1.193 g of HEPES was added to 100 mL of DI water to achieve the concentration of 50 mM. The HEPES solution was sterilized using the same vacuum filtration method in a sterile laminar flow hood.
Preparation of NaCl solution
Preparation of MgCl2 solution
Is 2mg O2 2mgo A Decomposition Reaction
For example, calcium carbonate on heating decomposes to form calcium oxide and carbon dioxide. For example, magnesium reacts with air to form magnesium oxide. Therefore, the given reaction $2Mg+_}\to 2MgO$ is an example of combination as well as oxidation reaction. So, the correct answer is Option C and D.
Recommended Reading: Beth Thomas Rad
What Is Magnesium Oxide
Magnesium oxide , or magnesia, is a white hygroscopic solid mineral that exists in nature as periclase and is a source of magnesium. It has an empirical formula of MgO and consists of a Mg2+ ions and O2 ions held together by ionic bonding. In the presence of water, magnesium hydroxide is formed 2), but it may be possible to remove moisture by heating.
Magnesium oxide was historically known as magnesia alba, to distinguish it from magnesia negra, a black mineral containing what is now known as manganese.
Magnesium oxide normally stands for MgO, while magnesium peroxide MgO2 is also known as a compound. According to the evolutionary crystal structure prediction, MgO2 is thermodynamically stable at pressures above 116 GPa , and a semiconductor suboxide Mg3O2 is thermodynamically stable above 500 GPa. Because of its stability, MgO is used as a model system to investigate the vibrational properties of crystals. In addition, it is used in many different sectors and fields as a material that is highly resistant to high temperatures.
What Is Magnesium Oxide In Chemistry
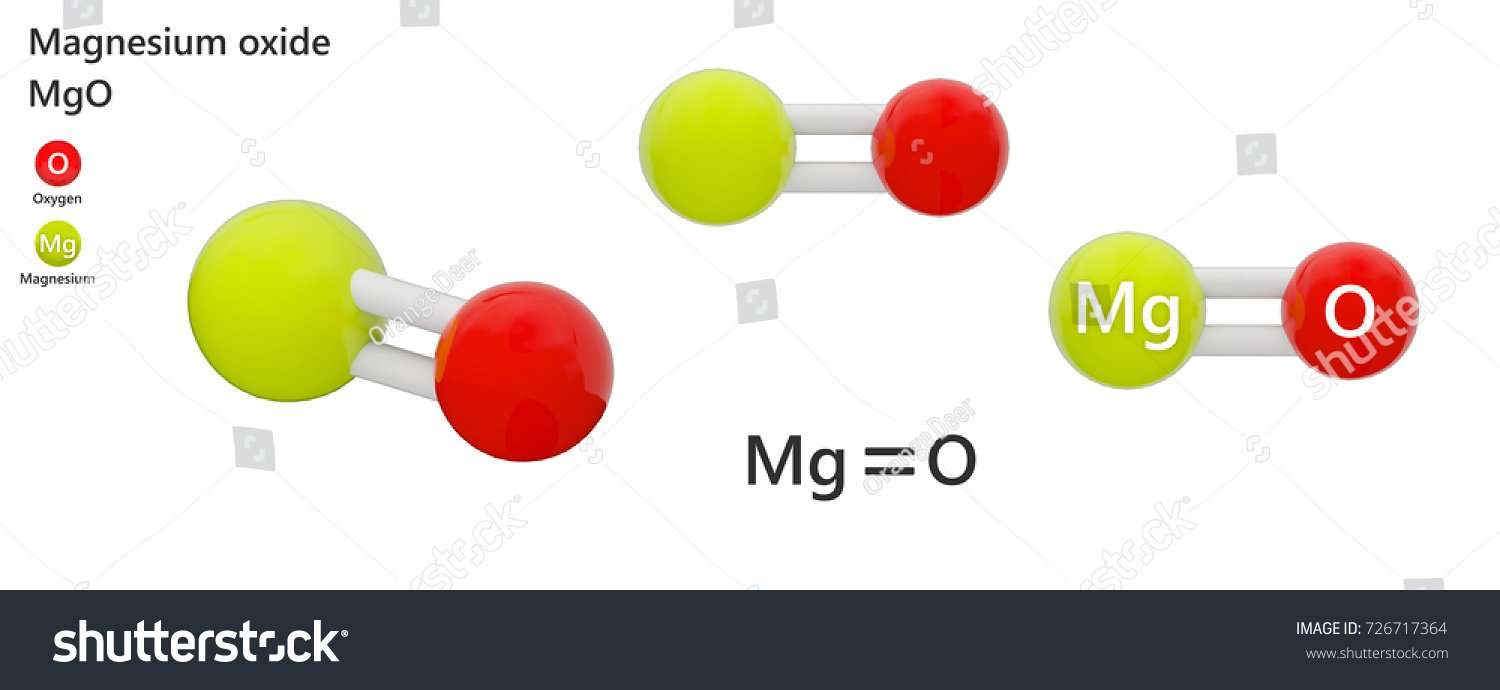
Magnesium oxidemagnesium
. Besides, how Magnesium oxide is formed?
Oxygen and magnesium combine in a chemical reaction to form this compound. After it burns, it forms a white powder of the magnesium oxide. Magnesium gives up two electrons to oxygen atoms to form this powdery product. This is an exothermic reaction.
Also Know, what is the composition of magnesium oxide? The actual % composition by mass of magnesium oxide is: 60% magnesium, 40% oxygen.
Subsequently, one may also ask, is magnesium oxide a metal?
No it is not. When elements combine to form compounds they do this chemically and so therefore the properties of the metal and the oxide will change. What mass of oxygen is required to react with 5.0g of magnesium to form magnesium oxide?
Why is magnesium oxide bad?
* Magnesium Oxide can affect you when breathed in. * Breathing Magnesium Oxide can irritate the eyes and nose. * Exposure to Magnesium Oxide can cause metal fume fever. This is a flu-like illness with symptoms of metallic taste in the mouth, headache, fever and chills, aches, chest tightness and cough.
Recommended Reading: Difference Between Electron And Molecular Geometry