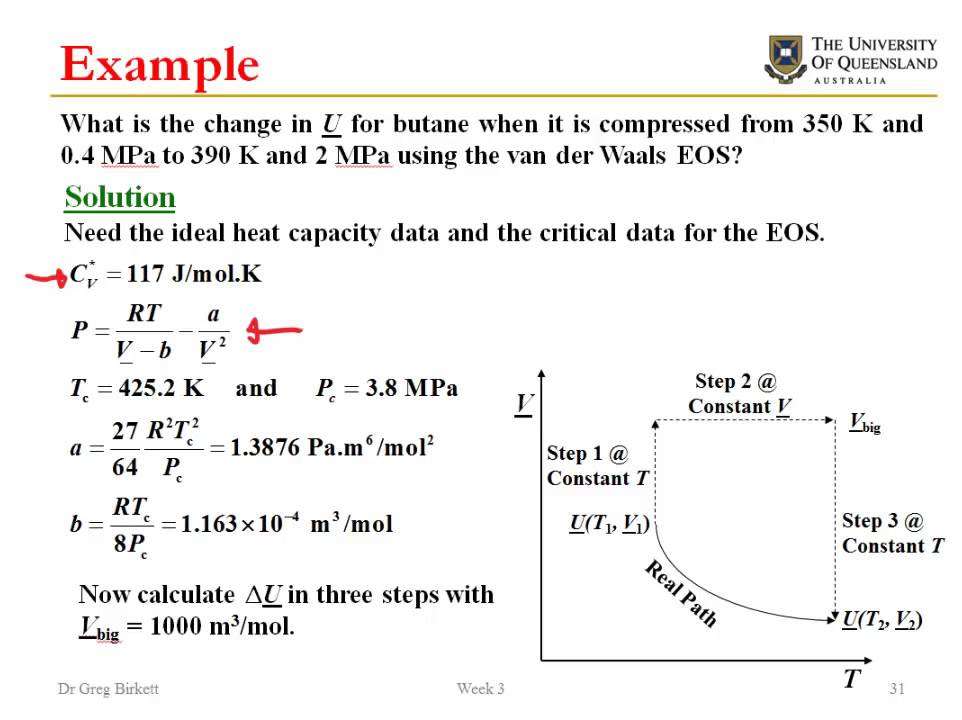
How To Calculate Bond Energy
This article was co-authored by Bess Ruff, MA. Bess Ruff is a Geography PhD student at Florida State University. She received her MA in Environmental Science and Management from the University of California, Santa Barbara in 2016. She has conducted survey work for marine spatial planning projects in the Caribbean and provided research support as a graduate fellow for the Sustainable Fisheries Group.There are 8 references cited in this article, which can be found at the bottom of the page. This article has been viewed 208,402 times.
Bond energy is an important concept in chemistry that defines the amount of energy needed to break a bond between a covalently bound gas.XResearch source This type of bond energy does not apply to ionic bonds.XResearch source When 2 atoms bind together to form a new molecule, it is possible to determine how strong the bond between atoms is by measuring the amount of energy needed to break that bond. Remember, a single atom does not have a bond energy it is the bond between 2 atoms that has energy. To calculate the bond energy of a reaction, simply determine the total number of bonds broken and then subtract the total number of bonds formed.
The Three Laws Of Thermodynamics
Before we tell you about the three laws of thermodynamics, we should mention what they refer to. When scientists refer to these laws, theyre talking about a system and its surroundings. The surroundings are basically just everything that isnt part of the system.
The system is separated from the surroundings by some sort of a boundary. This can be the wall of the container, for example, if the system is all of the molecules that are in the container. Closed systems are systems where matter is not able to pass between the system and surroundings , while open systems allow for this exchange of matter .
First law
The first law of thermodynamics is the law of conservation of energy. It states that energy cant be created or destroyed within a system. It can only be transferred or converted from one form of energy to another.
There are two processes that can lead to a system experiencing a change in internal energy. These would be heat and work. The system could do work on the surroundings, or heat could flow into the system from the surroundings.
In either of these cases, energy isnt being created or destroyed. Its just being transferred and transformed between the system and surroundings.
Second law
According to the second law of thermodynamics, the entropy of any isolated system is always going to be increasing. Its a spontaneous process. Isolated systems are always going to be heading toward thermal equilibrium, which equals the maximum entropy that a system can have.
Endothermic Or Exothermic Reaction
There are two main types of thermodynamic reactions: endothermic and exothermic. An endothermic reaction causes absorption of heat from the surroundings. An exothermic one releases heat to the surroundings.
Both these reaction types cause energy level differences and therefore differences in enthalpy. All you need to remember for the purpose of this calculator is:
- If the reaction is endothermic, the change in enthalpy is positive, as heat is gained .
- If the reaction is exothermic, the change in enthalpy is negative, as heat is lost .
Recommended Reading: Hawkes Learning Systems Statistics Answer Key
How To Calculate The Enthalpy Of A Reaction
The enthalpy calculator has two modes. You can calculate the enthalpy change from the reaction scheme or by using the enthalpy formula. If you select the former:
If you want to calculate the enthalpy change from the enthalpy formula:
Begin with determining your substance’s change in volume. Let’s assume your liquid expanded by 5 liters.
Find the change in the internal energy of the substance. Let’s say your substance’s energy increased by 2000 J.
Measure the pressure of the surroundings. We will assume 1 atmosphere.
Input all of these values to the equation H = Q + p * V to obtain the change in enthalpy:
H = 2000 J + 1 atm * 5 l = 2000 J + 101,325 Pa * 0.005 m³ = 2506.63 J
You can also open the advanced mode of our enthalpy calculator to find the enthalpy based on the initial and final internal energy and volume.
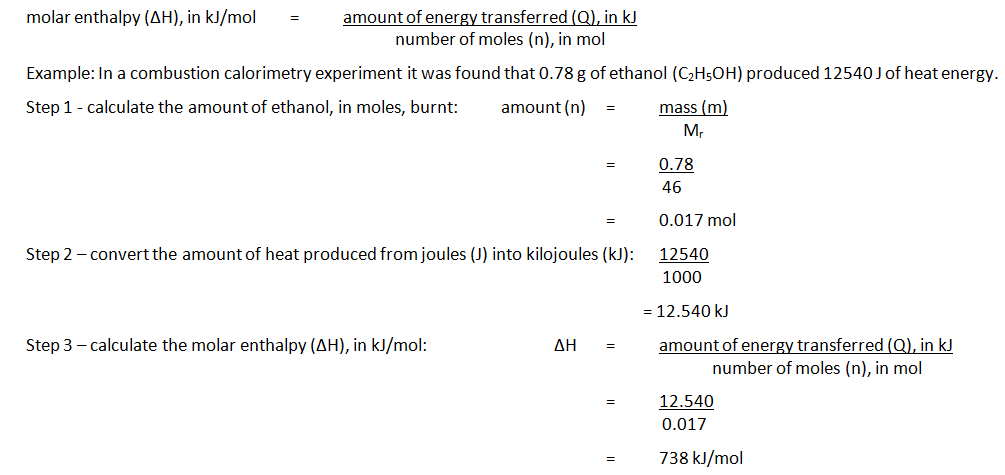
Observing Enthalpy Changes Experimentally
Also Check: Is Biology The Same As Living Environment
Standard Enthalpy Of Formation Table And Definition
For more particular problems, we can define the standard enthalpy of formation of a compound, denoted as H°f. It’s the change in enthalpy, H, during the formation of one mole of the substance in its standard state, ° , from its pure elements, f.
The standard enthalpy of formation formula for a reaction is as follows:
H°reaction = H°f – H°f
where:
-
H°reaction Standard enthalpy change of formation expressed in kJ
-
H°f Sum of the standard enthalpies of formation of the products expressed in kJ/mol and
-
H°f Sum of the standard enthalpies of formation of the reactants, expressed in kJ/mol.
If you’re paying attention, you might have observed that H°f and H°f have different units than H°reaction. This is because you need to multiply them by the number of moles, i.e., the coefficient before the compound in the reaction. We’ll show you later an example that should explain it all.
But before that, you may ask, “How to calculate standard enthalpy of formation for each compound?” The most straightforward answer is to use the standard enthalpy of formation table! Here’s an example one:
| Substance | |
|---|---|
| Mg² | -466.85 |
The symbols in the brackets indicate the state: s – solid, l – liquid, g – gas, and aq – dissolved in water. If you need the standard enthalpy of formation for other substances, select the corresponding compound in the enthalpy calculator’s drop-down list. We included all the most common compounds!
H°reaction = 2 mol * + 1 mol * 0 kJ/mol – 2 mol *
Entropy Changes In Chemical Reactions
— It’s most important to focus on the amount of gas molecules.
ex: Does entropy increase or decrease in the following reactions?
i. N2 + 3H2 2NH3
– 4 gas molecules “goes to” 2 gas molecules, and fewer gas molecules means fewer possible configurations.
– fewer gas molecules = less disorder = entropy decreases, S < 0
ii. 4NH3 + 5O2 4NO + 6H2O
– entropy increases, S > 0, because 9 gas molecules becomes 10 gas molecules.
=====
Also Check: Unit 1 Study Guide Geometry Basics Answer Key
How To Calculate Specific Heat Capacity
Tips To Calculate Delta H In Chemistry
Make sure to remember the following tips at all times. They will help you avoid the most common mistakes:
- Write down the equation of the reaction youre studying in a horizontal form. Youll be writing delta h or H precisely on the top of the arrow of your chemical equation.
- Then, carefully write down the rest of the information of the reaction in the respective positions to design an accurate Hesss diagram.
- Finally, ensure that the direction of the arrowheads in your diagram is correct. The opposite direction of your arrowhead will make the whole diagram wrong.
Read Also: Mcdougal Littell Geometry Worksheet Answers
How To Calculate Specific Heat
If you have problems with the units, feel free to use our temperature conversion or weight conversion calculators.
Edit: This Is Nearly Impossible Unless We Are Talking About The Change In Enthalpy Of The Reaction We Would Otherwise Have To Calculate The Ground
Using bond enthalpies
Explanation:
Assuming you meant the ENTHALPY change of the reaction it becomes clearer. As Truong-Son pointed out it would be a hassle to calculate using the Schrodinger equation if we are truly talking about the ENERGY change.
Given that we are talking about Enthalpy changes, we can use bond enthalpies from a table to solve this. I found my bond enthalpies in this booklet, table 11
We need to determine what bonds are broken and what bonds are formed. Bond breaking is endothermic- we need to put energy into breaking the bond so the value for #DeltaH#
Bond making is exothermic, meaning energy will be released to the surroundings and #DeltaH# will be negative.
From the diagram’s product side, we can see that the Hydrogen gas and the C-O double bond have vanished, so the respective bonds must have been broken in the first step!
Hence:Breaking a C-O double bond= #DeltaH=+745 kj mol^-1# *Breaking an H-H single bond= #DeltaH=+436 kj mol^-1#
If we wanted to be thorough, we could compare all the bonds on both the product and reactant side, but here we can see that there is no change in the Methyl ## groups so the “breaking and making” would cancel out, mathematically.
Anyway, on the product side, we now have the central carbon single bonded to a hydrogen, an oxygen and in turn that oxygen is bonded to a hydrogen. We have 3 new bonds that were not present in the reactant step.
We have formed the following bonds:
#745+436+++=-54#
Don’t Miss: Practice Workbook Mcdougal Littell Geometry Answers
Enthalpy Change When Chemical Equations Are Reversed
If a chemical equation is reversed, the sign of the H value is also reversed.
Consider the chemical reaction for the synthesis of ammonia gas ) from nitrogen gas ) and hydrogen gas ) as shown in the balanced chemical equation below:
NH3 synthesis: N2 + 3H2 2NH3 H = 92.4 kJ mol-1
The reaction is exothermic, energy is released during the reaction, so the enthalpy change term is negative . Because energy is released, we can think of energy as being a product of the chemical reaction. So we could write this equation with energy shown as a product of the reaction:
| 2NH3 N2 + 3H2 | H = +92.4 kJ mol-1 |
We can generalise and say that the sign of H for the forward reaction is the opposite sign of H for the reverse reaction.For any reaction:
Forward reaction: X Z H = +h kJ mol-1
Reverse reaction: Z X H = h kJ mol-1
Do you know this?
Play the game now!
Gibbs Energy In Equilibria
Let’s consider the following reversible reaction:
The following equation relates the standard-state free energy of reaction with the free energy at any point in a given reaction :
- \ = free energy at any moment
- \ = standard-state free energy
- R is the ideal gas constant = 8.314 J/mol-K
- T is the absolute temperature
- \ is natural logarithm of the reaction quotient
At equilibrium, G = 0 and Q=K. Thus the equation can be arranged into:
\} \label\]
with
- \ = standard free energy change
- \ = gas constant = 1.98 * 10-3 kcal mol-1 deg-10
- \ = is usually room temperature = 298 K
- \
The Gibbs free energy \ depends primarily on the reactants’ nature and concentrations term and the logarithmic term of Equation 1.11, respectively).
At equilibrium, \: no driving force remains
\} \label\]
\} \label\]
The equilibrium constant is defined as
\} \label\]
When \ is large, almost all reactants are converted to products. Substituting \ into Equation 1.14, we have:
Rearrange,
This equation is particularly interesting as it relates the free energy difference under standard conditions to the properties of a system at equilibrium .
| \ |
- \ = Faraday’s constant: 96,485 coulombs per mole of electrons
multiply the entire equation by \
which is similar to:
it can be concluded that:
Therefore,
Some remarks on the Gibbs “Free” Energy
Don’t Miss: Michael Jackson Kids White
Second Law Of Thermodynamics
2nd Law of Thermodynamics = the entropy of the universe is constantly increasing. In any spontaneous process, the entropy of the universe increases.
The change in entropy of the universe is given the symbol, Suniv
if Suniv> 0 , the process is spontaneous. if Suniv < 0 , the reverse process is spontaneous. if Suniv = 0 , the process has no tendency to occur .
Sometimes Ssys and Ssurr have opposite signs, and their magnitudes determine whether or not the overall process is spontaneous .
=====
Enthalpy And Chemical Reactions
Endothermic Reactions: In endothermic reactions the heat is added to the system as reactants convert to products, and so the sign of H is positive. Now the units of Hreaction often confuse students, as there are actually implicit units of mole in the denominator, where it is based on the stoichiometric coefficient of the chemical you are interested in. The easiest way to look at this is as an equivalence statement, where the enthalpy of reaction is related to the coefficient of each species.
Reactants + Heat Products, H> 0
Example:
The above equation is really saying 2599 kJ are being released for every 4 moles of CO2, 2 moles H2O, 2 moles C2H2 and 5 moles O2.
This gives four equivalence statements
4 mole CO2 = 2559kJ
You May Like: Beththomas
Temperature Dependence Of Spontaneity
As was previously demonstrated in this chapters section on entropy, the spontaneity of a process may depend upon the temperature of the system. Phase transitions, for example, will proceed spontaneously in one direction or the other depending upon the temperature of the substance in question. Likewise, some chemical reactions can also exhibit temperature dependent spontaneities. To illustrate this concept, the equation relating free energy change to the enthalpy and entropy changes for the process is considered:
The spontaneity of a process, as reflected in the arithmetic sign of its free energy change, is then determined by the signs of the enthalpy and entropy changes and, in some cases, the absolute temperature. Since T is the absolute temperature, it can only have positive values. Four possibilities therefore exist with regard to the signs of the enthalpy and entropy changes:
These four scenarios are summarized in Figure 1.
Figure 1.
Key Takeaways: Find Photon Energy From Wavelength
- The energy of a photo is related to its frequency and its wavelength. It is directly proportional to frequency and inversely proportional to wavelength.
- To find energy from wavelength, use the wave equation to get the frequency and then plug it into Planck’s equation to solve for energy.
- This type of problem, while simple, is a good way to practice rearranging and combining equations .
- It’s also important to report final values using the correct number of significant digits.
You May Like: Beth Thomas Child Of Rage Now