How To Calculate Mass Ratio
In chemistry, mass ratio, often called “percent composition by mass,” is the proportion of a particular molecule that consists of each that molecule’s constituent elements. For example, water consists of 11.1 percent hydrogen and 88.9 percent oxygen , meaning that a 1,000-gram sample of water consists of 111 g of H and 889 g of O .
This principle gives rise to the Law of Constant Composition, put forth by Joseph Proust in 1800: A given compound always has the same proportion by mass of its constituent elements. For instance, water always has exactly 8 grams of oxygen for every gram of hydrogen. Carbon dioxide always has 2.67 g of oxygen for every gram of carbon.
Calculating mass ratios is easy enough if you have access to a periodic table and the means to do basic algebra.
Say you want to calculate the mass ratio of sulfuric acid, H2SO4.
H2SO4 contains hydrogen , sulfur and oxygen . From the periodic table, you can see that the molar masses of these elements are:
H = 1.00
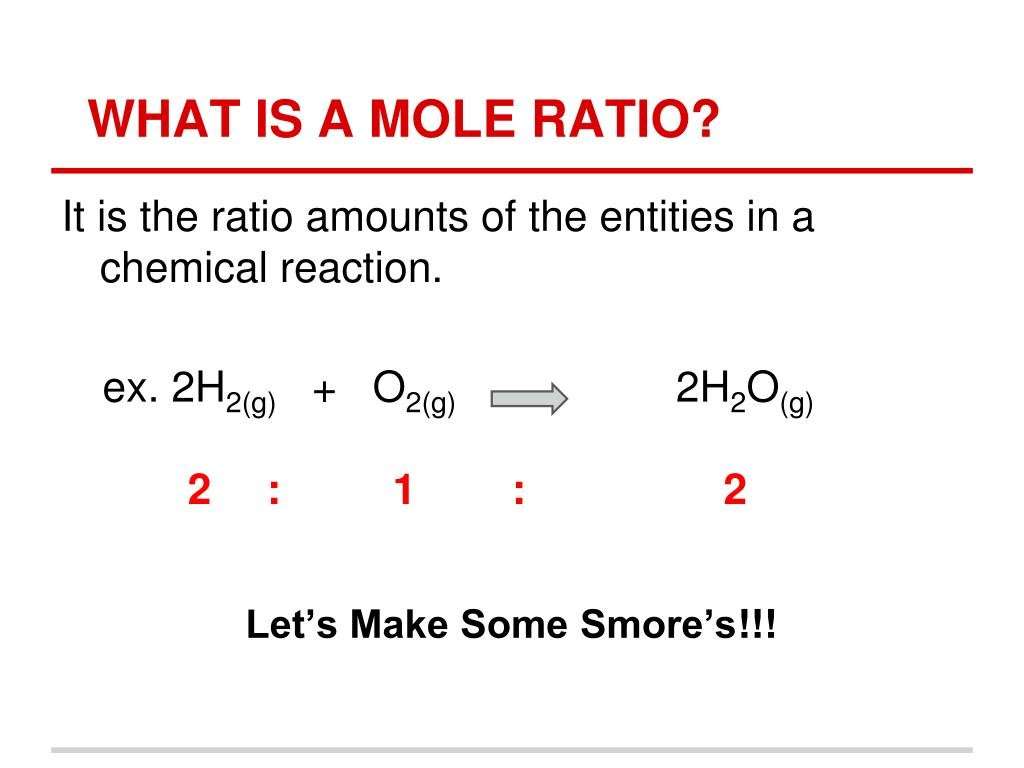
Mole Ratio Example: Balanced Equation
For the reaction:2 H2 + O2 2 H2O
The mole ratio between O2 and H2O is 1:2. For every 1 mole of O2 used, 2 moles of H2O are formed.
The mole ratio between H2 and H2O is 1:1. For every 2 moles of H2 used, 2 moles of H2O are formed. If 4 moles of hydrogen were used, then 4 moles of water would be produced.
How Much Ready To Use Solution Do You Need
Are you filling a bottle or a bucket or a tank?
Do you need 750ml? 6 litres? 50 litres?
Lets take an example of a bucket… Its an 11 litre bucket but you only want to fill it to just over half way, so lets say we need 6 litres in total of cleaning solution. Thats the ready to use solution you need.
Recommended Reading: Beth Thomas Brother
Measure Your Artresin Accurately
Measure accurately! Adding too much of either resin or hardener will alter the chemical reaction and the mixture will not cure properly. Example: Say you have a piece of artwork that is 2 feet x 3 feet. Entering 24 x 36 into the Resin Calculator gives you a result of 30 oz total resin required: this represents a combined volume of 15 oz of resin and 15 oz of hardener. Start by pouring 15 oz of resin into the measuring cup, followed by 15 oz of hardener, to give you 30 oz total. The 32 oz kit is the perfect amount for your projects needs.
Video advice: Mix Ratio Explained with EZ math
Dont let the math scare you anymore! This video shows a quick and easy way to calculate the ratios associated with mixing two-part resin systems like polyurethanes, epoxies, and silicones.
How To Find The Ratio Of Two Things
Question:
In a bag of 20 sweets, there are 8 blue sweets and 12 pink sweets. What is the ratio of blue to pink sweets?
Explanation:
This problem gives us all the information we need to express the ratio:
8 blue : 12 pink = 8:12
Remember, all ratios should be simplified where possible, so divide both the antecedent and consequent by the highest common factor in this case, the highest number that goes into both 8 and 12 is 4.
8 divided by 4 = 2
12 divided by 4 = 3
Therefore the correct answer is:2:3
Read Also: Geometry Warm Ups
Working With Ratios That Include Decimals
Question:
Simplify 10:2.5
Explanation:
To easily work with ratios, whole numbers are necessary. The easiest way to make this ratio include whole numbers is to multiply both sides by the same number in this case, 2 makes sense.
10 x 2 = 20
Our whole number ratio, therefore, will be 20:5
The highest common factor is 5 both sides can be equally divided by 5:
20 divided by 5 = 4
5 divided by 5 = 1
Therefore, the correct answer is:4:1
How To Measure And Mix Epoxy Resin And Hardener
Mixing the resin and hardener together prompts a chemical reaction between the two, transforming them from a liquid into a solid. Read our how to guide.
Sometimes people think they are able to accelerate solution time with the addition of more hardener towards the mixture. This will not work. Actually, itll mess up the fragile 1:1 mixing ratio as well as your resin wont cure. The easiest method to encourage curing would be to boost the 70 degrees, since curing is faster by heat.
Video advice: Mixing Ratio
Thermobytes: Atmospheric thermodynamics by Mick Pope, Australian Bureau of Meteorology
Don’t Miss: Is Michael Jackson The Biological Father Of His 3 Children
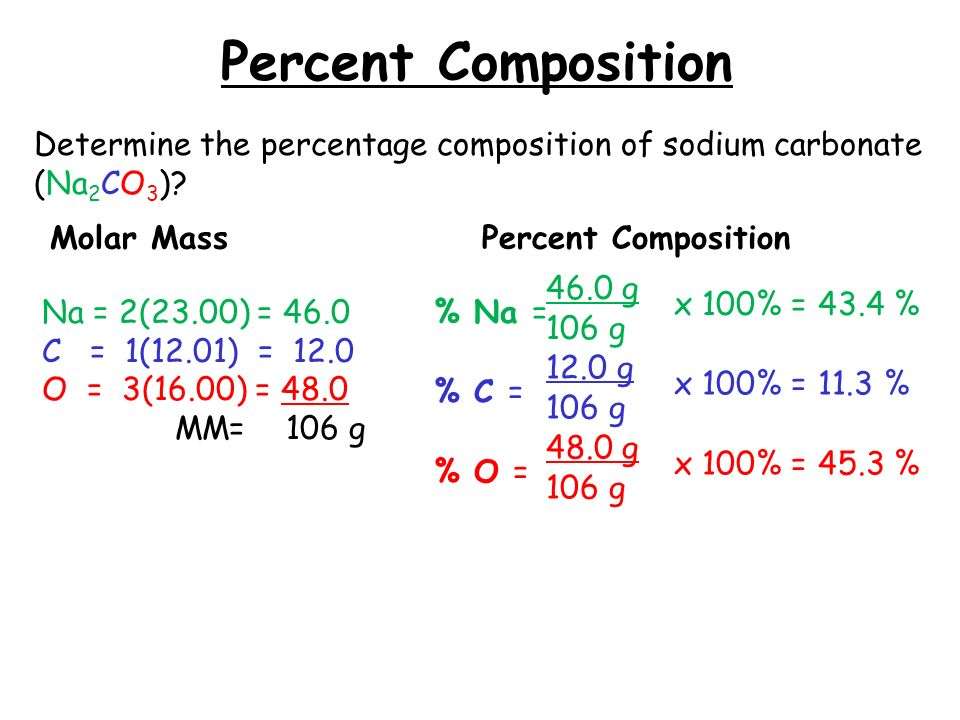
How To Calculate Molar Ratio
To determine the molar ratio between any two elements or compounds in a chemical reaction:
You can use the molar ratio and molecular weight of the molecules to determine the mass of the elements required to complete the reaction. Use our grams to moles calculator calculator and mole calculator to understand how molecular weight and molar mass of a compound are related and how to convert the number of moles to the required compound’s mass. Hence, once you know how to find the molar ratio and number the moles required, you can “convert” the molar ratio to grams or “mass” ratio:
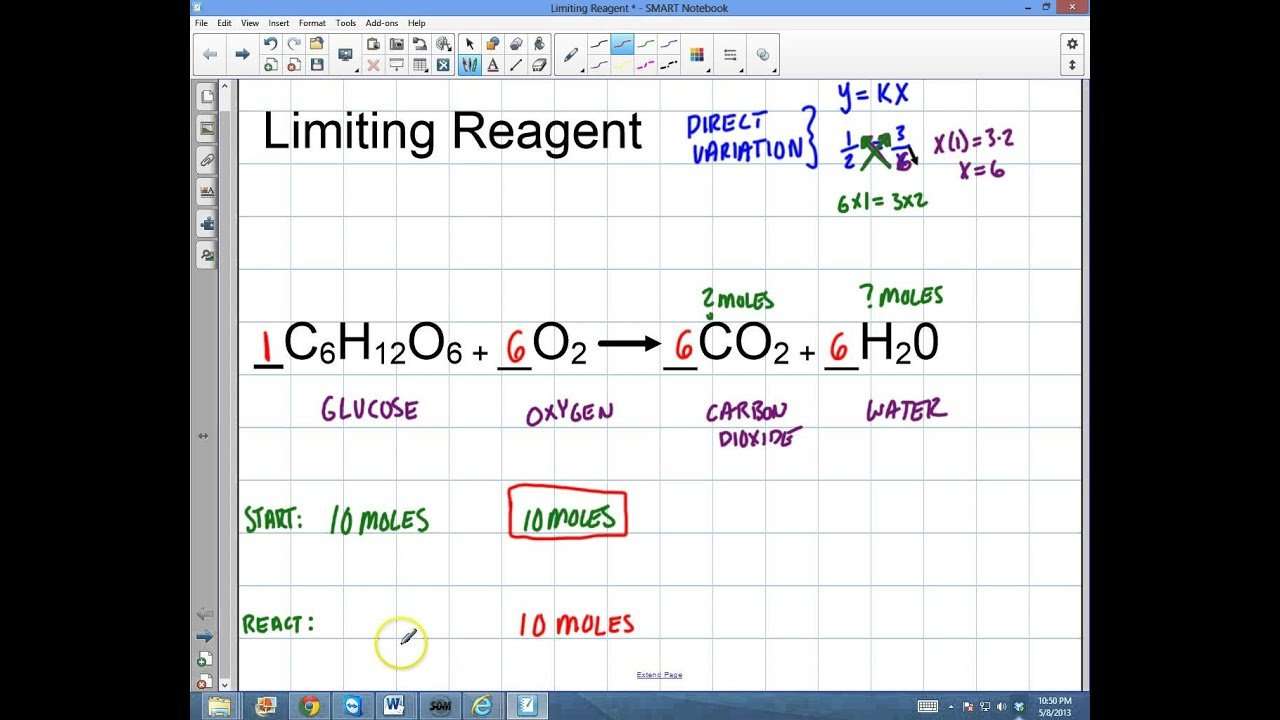
Since the molar ratio helps determine how much of each substance is required to complete the reaction, you can also use it to find out which substance is the limiting reagent and which substance is in excess. For example, we’ve seen that the molar ratio equation between hydrogen and oxygen in water production is 2:1. If we had 12 mol of hydrogen for 10 mol of oxygen, we immediately know hydrogen is the limiting reagent because the reaction demands that we have 20 mol of hydrogen. We can also phrase that oxygen is in excess by 4 mol since only 6 mol can react with 12 mol of hydrogen.
What Are The Similarities And Differences Between These Two Equations
One fundamental law of chemistry deals with the fact that we cannot create or destroy matter . When a reaction is run, the number of atoms of each specific type must be the same on both sides of the equation. For some materials, it turns out that one element can combine with a second element in more than one ratio. Carrying out mass ratio calculations helped establish the law of multiple proportions.
You May Like: Get The Message Algebra With Pizzazz
How To Locate Mole Ratio
12.2: Mole Ratios. Suppose that you want to add some sections to your porch. Before you go to the hardware store to buy lumber, you need to determine the unit composition . You count how many posts, how many boards, how many railsthen you decide how many sections you want to addbefore you calculate the amount of building material needed for your porch expansion.
Finally, if each mole quantity is converted to grams by using the molar mass, we can see that the law of conservation of mass is followed. \ of nitrogen has a mass of \, while \ of hydrogen has a mass of \, and \ of ammonia has a mass of \.
Video advice: Mole Ratios from Balanced Equations
Learn the SIMPLE way to write mole ratios. What are mole ratios? How do you write mole ratios? Why are mole ratios used in chemistry? In this video, you will learn how to write mole ratios from balanced equations and why mole ratios are used in chemistry. The concept of the mole, particles and bond energy in chemistry will be reviewed.
In stoichiometry, mole ratio is important when analyzing compounds and equation reactions. To analyze compounds, weigh the components and calculate the number of moles of each, using their atomic masses. In reactions you get the mole ratio of the reactants and products when you balance the equation.
- Determining Empirical Formula
- Balancing a Reaction Equation
Video advice: Determining the Mole Ratio
Determining the mole ratios of a balanced chemical equation for stoichiometry.
What Is The Recommended Chemical Concentrate Dilution Rate
Select the dilution rate that is appropriate for your task. You can look on the label or for more details check out the products training information guide for more details.
Lets continue with the example of the bucket that holds 6 litres of ready to use solution.
Now, if the chemical dilution rate you have selected is 1:32 how much water first needs to be added to the bucket and then how much chemical concentrate will you need?
Recommended Reading: Definition Of Span Linear Algebra
What Is A Molar Ratio What Is Molar Ratio Formula
A molar ratio is between the number of moles of reactants consumed and the number of moles of products generated in a chemical reaction. You can also express it as the ratio of the number of moles of one reactant required to completely react with another reactant or one product produced to another product.
For example, during ammonia production, if 30 moles of hydrogen react entirely with 10 moles of nitrogen to give 20 moles of ammonia, then you can write the molar ratio between different participants in the reaction as:
If you want to find how much of each element is present in a compound, use our percent composition calculator.
Formula Mass For Ionic Compounds
Ionic compounds are composed of discrete cations and anions combined in ratios to yield electrically neutral bulk matter. The formula mass for an ionic compound is calculated in the same way as the formula mass for covalent compounds: by summing the average atomic masses of all the atoms in the compounds formula. Keep in mind, however, that the formula for an ionic compound does not represent the composition of a discrete molecule, so it may not correctly be referred to as the molecular mass.
As an example, consider sodium chloride, NaCl, the chemical name for common table salt. Sodium chloride is an ionic compound composed of sodium cations, Na+, and chloride anions, Cl, combined in a 1:1 ratio. The formula mass for this compound is computed as 58.44 amu .
Figure 3.
Don’t Miss: How To Find Half-life Of A Reaction
How To Calculate Mass Percent Concentration Of A Solution
Mass percent composition is the easiest way to express the concentration of a solution because no unit conversions are required. Simply use a scale to measure the mass of the solute and the final solution and express the ratio as a percentage. Remember, the sum of all percentages of components in a solution must add up to 100%
Mass percent is used for all sorts of solutions but is particularly useful when dealing with mixtures of solids or anytime physical properties of the solution are more important than chemical properties.
Calculate Mass Percent: mass solute divided by mass final solution multiplied by 100%
symbol: %
Example: The alloy Nichrome consists of 75% nickel, 12% iron, 11% chromium, 2% manganese, by mass. If you have 250 grams of nichrome, how much iron do you have?
Because the concentration is a percent, you know a 100-gram sample would contain 12 grams of iron. You can set this up as an equation and solve for the unknown “x”:
12 g iron / 100 g sample = x g iron / 250 g sample
Cross-multiply and divide:
x= / 100 = 30 grams of iron
The Example In Detail
Each mole of carbon has a mass of 12.01g/mol of carbon. This, we know, from the periodic table. So, 6 moles of carbon will have 12.01g/mol x 6 = 72.06g of Carbon. Similarly, 1 mole of Hydrogen has a mass of 1.008g/mol of Hydrogen. Therefore, 12 moles of Hydrogen will have the mass of 12 x 1.008 = 12.096g of Hydrogen.
Going by the same logic for Oxygen, 1 mole of oxygen has a mass of 16.00g/mol. Therefore, 6 moles of oxygen will have 16.00 x 6 = 96 g of Oxygen. Thus, 1 mole of Glucose has a total mass of 72.06 +12.096 + 96 = 180.16 g/mol
Also Check: What Are The 4 Main Goals Of Psychology
How To Calculate Molarity Of A Chemical Solution
Molarity is one of the most common units of concentration. It is used when the temperature of an experiment won’t change. It’s one of the easiest units to calculate.
Calculate Molarity: moles solute per liter of solution
symbol: M
M = moles / liter
Example: What is the molarity of a solution of 6 grams of NaCl dissolved in 500 milliliters of water?
First, convert grams of NaCl to moles of NaCl.
From the periodic table:
- NaCl = 23.0 g/mol + 35.5 g/mol = 58.5 g/mol
- Total number of moles = * 6 g = 0.62 moles
Now determine moles per liter of solution:
M = 0.62 moles NaCl / 0.50 liter solution = 1.2 M solution
Note that I assumed dissolving the 6 grams of salt did not appreciably affect the volume of the solution. When you prepare a molar solution, avoid this problem by adding solvent to your solute to reach a specific volume.
Using Ratios To Work Out The Direct Proportions Of Quantity
Question:
If you go to the shop and buy 4 apples for £0.64, how much would 11 apples cost?
Explanation:
This might not look like a problem where ratios could help but considering this problem by expressing the given numbers as a ratio will help you to solve the problem.
The numbers that are directly proportionate increase in the same ratio.
Here, we know that 4 apples cost £0.64
£0.64 divided by 4 = £0.16
So, we know that 1 apple costs £0.16
You now have a ratio that you can use to find your answer:
1:16
To keep the proportions correct, we need to multiply both parts of the ratio by the required number in this case, 11.
1 x 11 = 11
Therefore, the correct answer is:11 apples cost 176p or £1.76
Recommended Reading: Does Kamala Harris Have Children
What Is A Ratio
The answer to the above question is yes, and this is where the concept of the ratio between two numbers becomes a part of your mathematical skill set even if you have no plans to become either a skier or a meteorologist.
A ratio is a kind a fraction, one whole number “over” another. This is the same basic operator as division, so a ratio is also a quotient. Examples are 1/3 and 8,298/27,209.
Sample Questions And Their Solutions
Understanding how to work out ratios is an important skill and can be particularly useful when applying for jobs where a good understanding of mathematics is required.
It is a good idea to revise skills like this before taking numerical reasoning or other math-based aptitude tests.
Here are the key ratio skills that you need to master:
You May Like: Are Michael Jackson’s Children Biologically His
How Do You Find Molar Ratio Using This Online Molar Ratio Calculator
This online molar ratio calculator can handle up to five reactants and five products. Additionally, there are three types of calculations to choose from, explained below. Keep in mind that in all these cases, the resultant molar ratio is displayed as a table at the very bottom of the calculator.
Check out our other calculators related to molecular weight and molar ratio:
More Ways To Calculate And Express Concentration
There are other easy ways to express the concentration of a chemical solution. Parts per million and parts per billion are used primarily for extremely dilute solutions.
g/L= grams per liter = mass of solute / volume of solution
F= formality = formula weight units per liter of solution
ppm = parts per million = ratio of parts of solute per 1 million parts of the solution
ppb= parts per billion = ratio of parts of solute per 1 billion parts of the solution.
You May Like: Michael Jackson’s Biological Son
How To Calculate And Solve For Volume Ratio
The image above represents volume ratio.
To compute for volume ratio, two essential parameters are needed and these parameters are Mass of the Liquid Metal filling the Mould Cavity, Gates and Risers and Casting Volume .
The formula for calculating volume ratio:
Kv = G / V
G = Mass of the Liquid Metal Filling the Mould Cavity,Gates and RisersV = Casting Volume
Lets solve an example Find the volume ratio when the mass of the liquid metal filling the mould cavity, gates and risers is 12 and the casting volume is 24.
This implies that
G = Mass of the Liquid Metal Filling the Mould Cavity,Gates and Risers = 12V = Casting Volume = 24
Kv = 0.5
Therefore, the volume ratio is 0.5.
Calculating the Mass of the Liquid Metal Filling the Mould Cavity, Gates and Risers when the Volume Ratio and the Casting Volume is Given.
G = Kv x V
G = Mass of the Liquid Metal Filling the Mould Cavity,Gates and RisersKv = Volume RatioV = Casting Volume
Lets solve an example Find the mass of the liquid metal filling the mould cavity, gates and risers when the volume ratio is 15 and the casting volume is 10.
This implies that
G = 150
Therefore, the mass of the liquid metal filling the mould cavity,gates and risers is 150.
Calculating the Casting Volume when the Volume Ratio and the Mass of the Liquid Metal Filling the Mould Cavity, Gates and Risers is Given.
V = Kv / G
G = Mass of the Liquid Metal Filling the Mould Cavity,Gates and Risers
This implies that
V = Kv / G
Finally, Click on Calculate
How To Calculate Molality Of A Solution
Molality is used to express the concentration of a solution when you are performing experiments that involve temperature changes or are working with colligative properties. Note that with aqueous solutions at room temperature, the density of water is approximately 1 kg/L, so M and m are nearly the same.
Calculate Molality: moles solute per kilogram solvent
symbol: m
m = moles / kilogram
Example: What is the molality of a solution of 3 grams of KCl in 250 ml of water?
First, determine how many moles are present in 3 grams of KCl. Start by looking up the number of grams per mole of potassium and chlorine on a periodic table. Then add them together to get the grams per mole for KCl.
- K = 39.1 g/mol
- KCl = 39.1 + 35.5 = 74.6 g/mol
For 3 grams of KCl, the number of moles is:
* 3 grams = 3 / 74.6 = 0.040 moles
Express this as moles per kilogram solution. Now, you have 250 ml of water, which is about 250 g of water , but you also have 3 grams of solute, so the total mass of the solution is closer to 253 grams than 250. Using 2 significant figures, it’s the same thing. If you have more precise measurements, don’t forget to include the mass of solute in your calculation!
- 250 g = 0.25 kg
- m = 0.040 moles / 0.25 kg = 0.16 m KCl
Don’t Miss: Algebra With Pizzazz Answer Key Page 34 Books Never Written