What Harm Do Detergent Chemicals Do
You might think this is a matter of opinion mostly it’s a matter of science: the effects of detergent chemicals are welldocumented. What’s less well understood is that all chemicals are added to detergents for a specific purpose, and some of the additives actually reduce the harmful impacts that detergents would otherwise have.
Surfactants
As we’ve already seen, these play a crucial part in helping water to attack and remove dirt.But once they flush away down the drain, surfactants don’t stop working: they start to play similar trickson aquatic life, for example, attacking the natural oils in the mucus membranes of fish, stopping their gills from working properly, and increasing their risk of attack from other chemicalsin the water. Some surfactant ingredients produce what are calledendocrine-disruptors, which can affect the hormonal balance of animals . Although surfactants can be toxic to fish and other aquatic life ones that remain in the environment for many years without breaking down), most surfactants biodegrade relatively quickly in sewage treatment plants before they can domuch harm to the natural world.
Phosphates
Enzymes
Perfumes
Fragrances in detergent serve no purpose other than to make your clothes smell nice. But the oils from which they’re made cancause rashes and skin allergies.
Manufacturing Process Of Soap
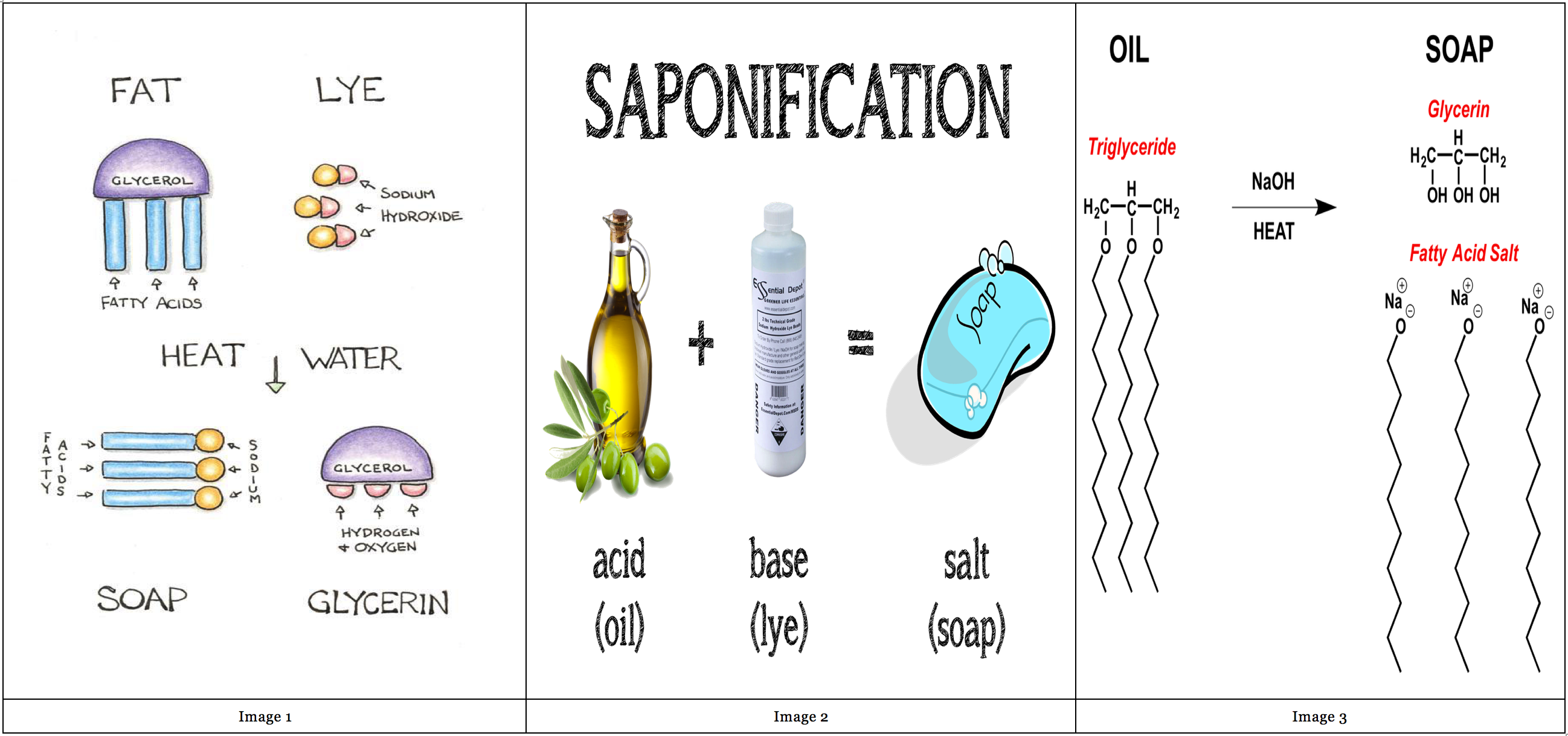
Both fats and oils are needed to make soaps and they are extracted from animals and plants. For making the fatty acid molecules like those of triglycerides, three molecules of fatty acids get added to one molecule of glycerine. These fatty acids are weaker and consist of two different parts. One is a carboxylic group which consists of one hydrogen atom, two oxygen atoms and one carbon atom. The other is a hydrocarbon chain which is attached to the carboxylic acid group. Generally, a soap is created from a long chain of the carbon atoms that carry two hydrogen atoms. Previously, the alkali which was needed to make soaps was derived from animals, but today it is clinically composed. The common alkalis that are used to make soaps are sodium hydroxide, which is commonly called as caustic soda, and potassium hydroxide, which is commonly called as caustic potash.
The manufacturing process of soaps consists of the following different methods.
Advantages Of Soaps And Detergents
The advantages of Soaps & detergents are discussed below:
You May Like: Which Founding Contributors To Psychology Helped Develop Behaviorism
Soap Manufacturing Processes And Products
Hot caustic alkali solution, such as caustic soda , acts on natural fats or oils, such as tallow or vegetable oil, to produce sodium fatty acid salt and glycerin . This saponification reaction is the basis for all soapmaking. If industrially produced fatty acids are used in place of natural fats or oils, the reaction with caustic soda yields soap and water instead of soap and glycerin.
The Disadvantage Of Soap
Although soaps are excellent cleansers, they do have disadvantages. As salts of weak acids, they are converted by mineral acids into free fatty acids:
CH316CO2-Na++ HCl â CH316CO2H + Na++ Cl-
These fatty acids are less soluble than the sodium or potassium salts and form a precipitate or soap scum. Because of this, soaps are ineffective in acidic water. Also, soaps form insoluble salts in hard water, such as water containing magnesium, calcium, or iron.
2 CH316CO2-Na++ Mg2+ â 2Mg2++ 2 Na+
The insoluble salts form bathtub rings, leave films that reduce hair luster, and gray/roughen textiles after repeated washings. Synthetic detergents, however, may be soluble in both acidic and alkaline solutions and don’t form insoluble precipitates in hard water. But that is a different story…
Recommended Reading: Geometry Segment Addition Postulate Worksheet
The Chemistry Of Soaps Shampoos And Laundry Detergents
Soaps, shampoos, and laundry detergents are mixtures of ingredients . The surfactants are the essential cleaning substances and they determine the cleansing and lathering characteristics of the soap, as well as its texture, plasticity, abrasiveness, and other features. Surfactants are compounds that have a dual affinity: They are both lipophilic and hydrophilic . A surfactant molecule consists of a lipophilic tail group, which links to greasy soil, and a hydrophilic and polar head group, which renders the molecule water-soluble this arrangement helps to disperse and rinse away greasy soil. Variations in the balance between hydrophobic and hydrophilic features determine the use of the surfactant as a detergent, wetting agent , or emulsifier .
Surfactants are classified according to the nature of the hydrophilic head. There are four main classes: anionic, cationic, amphoteric, and nonionic. The first three refer to charged surfactant molecules. An anionic surfactant possesses a negative charge and needs to be neutralized with an alkaline or basic material in order for its full detergent capacity to be realized, whereas a cationic surfactant is positively charged and needs to be neutralized by an acid. Amphoterics include both acidic and basic groups, and nonionics contain no ionic constituents. “Natural” soap contains an anionic surfactant. The majority of surfactants that are used in personal cleansing bars and shampoos have anionic head groups.
What Are Soaps And Detergents
Soaps and detergents are known as the chemical compounds of a mixture of compounds that are used as cleansing agents. A soap is either a sodium or a potassium salt of different combinations of fatty acids that possess cleansing action in water. On the other hand, detergents are far better solutions when it comes to cleaning purposes since they are not affected by the hardness of the water.
You May Like: Lesson 4.5 Practice B Geometry Answers
What Effect Do Detergents Have On The Environment
We all love clean clothes, but most of us also love a clean planet.Do the two things go together? Look at the ingredients label on a typical bottle of detergent and you’ll seea chemical cocktail. What are all these things and what do they do? Moreto the point, do they have any harmful effect on our health or the planeton which we all depend? There’s very good reason to think so.That’s why some detergent brands deliberately position themselves as eco-friendly, not by comparing themselvesto soap and water but bydrawing attention to the potentially harmful chemicals used by their rivals.
How Do Soaps And Detergents Clean Out Dirt
Cleaning a soiled surface is a four-step process. In the first step, the surface to be cleaned is made wet with water. In the second step, soap or detergent is applied to the surface to be absorbed.
Soaps and detergents are also called surface-active agents, or surfactants. Surface active molecules present in soaps and detergents dissolve in water. This solution serves to loosen surface tension or the force that holds together molecules on a surface or on cloth. When this happens, it helps water to spread easily over a surface or soak into clothes.In the third step, when clothes are rubbed together, either by hand or in a washing machine, dirt particles are broken up as surface-active molecules work to separate the dirt from clothes and deposit them in the water. In the fourth and final step of the cleaning process, the separated dirt is prevented from going and re-depositing on the surface of clean clothes. Dirt particles are coated with soap and detergent molecules. This keeps them suspended in water until the dirt is washed away with rinsing.
Read Also: What Does Abiotic Mean In Biology
Cleansing Action Of Detergents
A molecule of any detergent is made up of the following two parts:
Cleansing Action of Detergents
The polar end of a detergent molecule is water-soluble, whereas the hydrocarbon part is water repellent and oil soluble. When an oily piece of cloth is dipped into a detergent solution, the Detergents hydrocarbon end bonds to the oily drop and the polar end orients itself towards the water, resulting in the production of a micelle. The oily dirt is entrapped by the negatively charged micelle that has formed. The ions in the solution arrange themselves around the micelles. The negatively charged micelles repel each other due to the electrostatic repulsion. As a result, the tiny oily dirt particles do not come together and get washed away in the water.
How Do Detergents Work
Soaps and detergents are made from long molecules that contain a head and tail. These molecules are called surfactants the diagram below represents a surfactant molecule.
The head of the molecule is attracted to water and the tail is attracted to grease and dirt . When the detergent molecules meet grease on clothes, the tails are drawn into the grease but the heads still sit in the water.
The attractive forces between the head groups and the water are so strong that the grease is lifted away from the surface. The blob of grease is now completely surrounded by detergent molecules and is broken into smaller pieces which are washed away by the water. You can find out more about how detergents work here.
The detergent molecules also help to make the washing process more effective by reducing the surface tension of the water. Surface tension is the force which helps a blob of water on a surface hold its shape and not spread out. The surfactant molecules of the detergent break apart these forces and make water behave, well, wetter!
Bubbles and soap films are made of a thin layer of water, sandwiched between two layers of soap molecules. You can make giant bubbles by mixing these ingredients together:
- 1 litre of water ,
- 15 ml good quality washing-up liquid ,
- 10 ml glycerol/glycerine .
Once you’ve made your bubble solution, you can try our four experiments!
Don’t Miss: What Does K Stand For In Math
How To Select The Best Detergent
There are dozens of choices on the laundry detergent shelves. How do you choose? The best choice is the one that suits your familys needs in terms of effectiveness on specific soils, personal preference for fragrance, form , and price.
Here’s how to start. Assess your family’s laundry including the types of stains and the amount of body soil. If most of the garments are only lightly-soiled with few stains, you may find that a less expensive detergent and a good stain remover is all you need. If you have heavy soil, gym clothes with lots of body odor and lots of food/grease/outside stains you need a heavy-duty detergent.
Next, read the laundry detergent labels or go online to read the ingredients. It is important to look for surfactants and enzymes to remove soil and stains. Bargain brands have fewer of these components and will not clean as well. You may find that having two formulas on your laundry shelf will serve your needs one detergent for lightly soiled clothes and one for heavily-soiled clothes.
Although most detergents will work in cold water, it is better to choose one formulated for cold water if you plan to use cold water exclusively.
Many people chose their laundry detergent based on scent. Just remember that “smelling clean” is not the same as being clean. Be sure that soil is actually being removed and not just covered up with perfume.
The Chemistry Of Soap Making

Lucy Bell-Young
The chemistry of soap making is an ancient science. In fact, soap is one of the earliest inventions of humanity. Its almost as old as civilisation, with its earliest recorded evidence being traced all the way back to ancient Babylon 4,800 years ago though its invention probably dates back much farther than this. Even more astounding is that the basic ingredients of soap havent changed over the millenia so how is it made exactly?
In this post:
Read Also: What Is Biomass In Biology
Appliance Science: The Clean Chemistry Of Laundry Detergents
How do your clothes go from filthy to grunge free? Through the chemistry of laundry detergent. In the latest installment of our Appliance Science column, we look at the chemistry of clean clothes.
There’s one thing that most people don’t realize about the past: it was a filthy, filthy place. Even ignoring the people themselves, the clothes that we have worn for most of human history have been stained, dusty and crusted with all sorts of unpleasantness. We’ve worn them for a long time, getting them sweaty, dirty and generally covered in all sorts of icky stuff. Fortunately, much of humanity doesn’t have that issue anymore, thanks to the discovery of laundry detergents. Today, we can throw some socks in a washing machine and have them clean in a few minutes, thanks to the remarkable efficiency of laundry detergents at getting the dirt out of your clothes.
Modern laundry detergents contain a huge array of chemicals that help the cleaning process and make your clothes look nice, including chemicals that digest stains, clean the water and perform many other tasks. We’ll discuss more of these in future columns, but for right now, let’s focus on the two main ingredients of all laundry detergents: the water conditioners and the detergent that does the dirty work.
What Is The Chemical Name For Soap
Just like other substances, such as sugar, the chemical name of a particular type of soap depends on its composition. Here are some of the most common types and the respective chemical names of soaps:
- Sodium tallowate: This is made using sodium hydroxide and tallow, i.e. beef fat or mutton fat
- Sodium palmate: This is made using sodium hydroxide and palm oil as the main ingredients
- Sodium palm kernelate: This is made with sodium hydroxide and palm kernel oil
- Sodium cocoate: This is made using sodium hydroxide and coconut oil
Read Also: Is Paris Michael Jackson Biological Child
What Is The Difference Between Soap And Detergent
Soaps are the sodium salts of carboxylic acids in long chains. Sodium salts of long-chain benzene sulphonic acids are detergents. Soaps are biodegradable while some of the detergents can not be biodegraded. Soaps have relatively weak cleaning action, whereas detergents have a strong cleaning effect.
Laundry Detergent Ingredients And How They Work
The Spruce / Michelle Becker
Laundry detergents have come a long way since the first bar soaps made from animal fat and lye were offered for sale in the 1700s. The introduction of synthetic detergents to the marketplace in the 1950s offered homemakers more options for fabric care. But it was the 1970s that brought the most significant innovation in the laundry, the addition of enzymes that “attack” specific types of stains. It is those enzymes that separate the men from the boys when it comes to clean laundry.
Read Also: Angles And Angle Addition Postulate Worksheet Answers
How Detergents Work To Clean Clothes
To get the best results from any laundry detergent, there is a three-fold process of chemical energy, thermal energy, and mechanical energy that must be used when washing clothes.
The chemical energy is, of course, the laundry detergent. The ingredients in the laundry detergent you choose will affect the final results. Less expensive detergents have fewer or no enzymes. Fewer enzymes equal less cleaning power.
Thermal energy pertains to water temperature. Different detergents are formulated to work best at different temperatures. Be sure to read the directions to select the best product for your laundry.
Mechanical energy comes from either a washer or a person hand-washing clothes.
What Is A Surfactant
Surfactants change how water behaves. When a surfactant is added, the surface tension is reduced. Now water can spread out and wet the surface we are trying to clean.
Now lets look at what happens on the surface.
Every surfactant has two ends. One end wants to be in water and the other does not.
The water-fearing end is known as the hydrophobic end. Hydrophobic comes from two Greek roots, hydro- and -phobia . Have you heard the phrase oil and water dont mix? This is important here!
The water-fearing end of the surfactant is made up of hydrocarbon chains. A hydrocarbon is a molecule that is made of hydrogen and carbon. The chains love oil and grease and will try to stay away from water.
The water-loving end is known as the hydrophilic end. We learned hydro- is a Greek root meaning water. So, if -phobic means fearing, then -philic means loving. The water-loving end of the chemical is attracted to water.
How these two ends interact with soil and water is the secret to how a surfactant works.
You May Like: What Does And Stand For In Math
Sequestering Or Chelating Agents
EDTA or its sodium salt has the property of combining with certain metal ions to form a molecular complex that locks up or chelates the calcium ion so that it no longer exhibits ionic properties. In hard water, calcium and magnesium ions are thus inactivated, and the water is effectively softened. EDTA can form similar complexes with other metallic ions.
Water-insoluble minerals such as talc, diatomaceous earth, silica, marble, volcanic ash , chalk, feldspar, quartz, and sand are often powdered and added to soap or syntheticdetergent formulations. Abrasives of an organic nature, such as sawdust, are also used.