What Is The Specific Heat Of Water How Is It Special
If youve ever walked along a beach on a sunny day and dipped your toes in the water to cool them off after the hot sand, youve taken advantage of the specific heat of water.
Despite how it may sound, specific heat doesnt refer to the exact temperature of something. Its a larger scientific concept that has to do with the energy it takes to heat a substance up. As you might have noticed from the example, not all substances warm up at the same ratehence the different temperatures of the sand and water.
Waters specific heat is one of its most interesting characteristics. In this article, well be covering what specific heat is, what equation you use to find specific heat, and why waters specific heat is so high.
The stove, pot, water, and steam all have different specific heats.
How To Calculate Specific Heat Capacity
High Specific Heat Of Water And Other Examples
For reference, Figure 1 below compares the specific heat of water with other common substances.
| Substance | |
| Ethyl alcohol | 2.4 |
Figure 1. This table compares water with several common substances in terms of their specific heat.
Because water has a high specific heat capacity, it takes a lot of energy to create temperature changes. It’s why coffee takes a long time to cool down, or why “a watched pot never boils.” It’s also why it takes a long time for the environment to respond to external changes.
When a specific quantity of excess carbon dioxide is added to the atmosphere, for example, it takes time for warming impact on the air, land, and ocean to become fully apparent. Even if there were a means to directly add heat to the Earth , it would take time for the temperatures to rise.
This means that the ocean can absorb a significant amount of heat before its temperature increases significantly. Similarly, when an external source of energy is removed, the ocean responds slowly and its temperature will not begin to fall immediately.
Put simply, the high specific heat capacity of water allows it to maintain a stable temperature, which is very crucial in sustaining life on Earth.
Don’t Miss: What Is The Value Of Kw In Chemistry
Specific Heat Capacity Of Natural Gas
The specific heat capacity at constant volume and pressure of an uniform compressible system can be respectively defined as ,
where, u is the internal energy of system, kJ/kmol h is the enthalpy of system, kJ/kmol.
It is known from the laws of thermodynamics for an ideal gas that,
For a real gas, the applicable relationship is,
For an ideal gas component i, the specific heat capacity at constant pressure can be derived as follows,
where, Bi, Ci, Di, Ei, Fi are constants related to component i.
For an ideal gas mixture,
For a real gas, the specific heat capacity at constant volume can be calculated by,
Substituting the EOS of natural gas into Eq. can yield the specific heat capacity at constant volume . According to the relation between cv and cp ), the specific heat capacity at constant pressure can also be easily calculated.
Table 4. Calculation formulas for specific heat capacity of natural gas.
| EOS | Calculation formulas for specific heat capacity |
|---|---|
| RK | |
| 2 |
The ratio of the specific heat capacity at constant pressure to that at constant volume is called the heat capacity ratio,
What Is The Formula For Specific Heat
The formula for specific heat capacity, C, of a substance with mass m, is C = Q /. Where Q is the energy added and T is the change in temperature. The specific heat capacity during different processes, such as constant volume, Cv and constant pressure, Cp, are related to each other by the specific heat ratio, = Cp/Cv, or the gas constant R = Cp – Cv.
Read Also: What Does S Mean In Physics
We Can Perform An Experiment To Measure The Specific Heat Of Substances Using The Change In Water Temperature
A method called calorimetry can be used to determine the specific heat of a substance or object.
Calorimetry can be summed up in four basic steps:
Bring the substance’s temperature up to a predetermined level.
Put this substance in a thermally insulated container with water with a known mass and temperature.
Allow the water and the substance to reach equilibrium.
Take the temperature of both when they are in equilibrium.
Because the container is thermally insulated, heat energy is transferred only to the water and not to the surrounding environment. As a result, the heat transmitted from the item equals the heat absorbed by the water.
With this, we can use the formula to write this heat transfer in terms of the following formula to solve for the specific heat of the substance or object.
Where:
mois the mass of the object
mwis the mass of the water
cois the specific heat of the object
cw is the specific heat of the water
Teqis the temperature at equilibrium
Thot is the initial temperature of the object
Tcold is the initial temperature of the water
How Is Heat Capacity Used
Heat capacity, also known as thermal capacity, is a physical property of matter defined as the amount of heat needed to cause a unit change in temperature in a given mass of material. The molar heat capacity is determined by dividing the heat capacity by the sum of substances in moles.
Join BYJUS for the most simplified approaches to your problems. Make learning simple.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
Also Check: What Does The Difference In Math Mean
Discover And Learn What Students Are Asking
?In Exercises 1-20, find the indefinite integral.\?In Exercises 1-4, plot the points in the same three-dimensional coordinate system. ?In Exercises 17 – 20, find z = f and use the total differential to approximate the quantity.\^}-\sqrt+?In Exercises 19 – 22, use the gradient to find the directional derivative of the function at P in the direction of v.\=x y, \quad P is a liquid, and Ar is a gas. List these substances in order of increasing inte
Heat Capacity And Specific Heat
- Page ID
- 53872
If a swimming pool and wading pool, both full of water at the same temperature, were subjected to the same input of heat energy, the wading pool would certainly rise in temperature more quickly than the swimming pool. The heat capacity of an object depends both on its mass and its chemical composition. Because of its much larger mass, the swimming pool of water has a larger heat capacity than the wading pool.
Don’t Miss: How To Get Experience In Clinical Psychology
What Is The Specific Heat Of Water
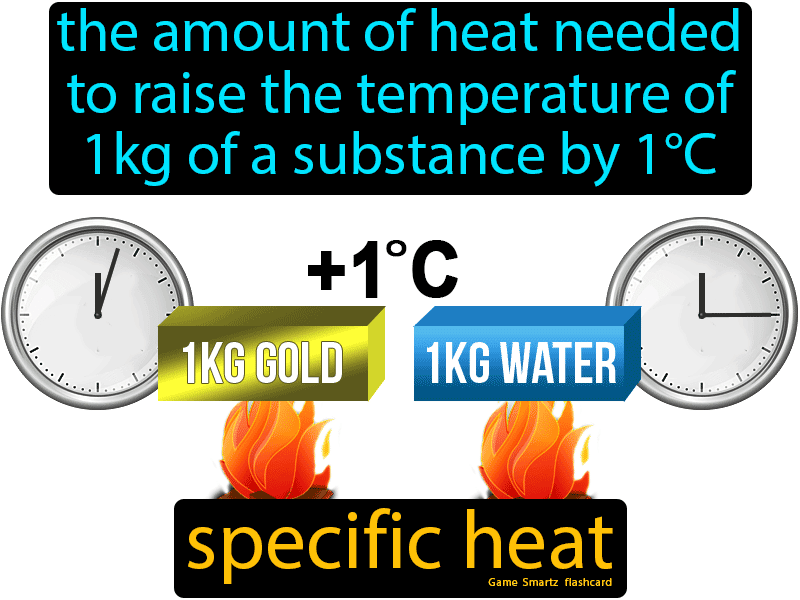
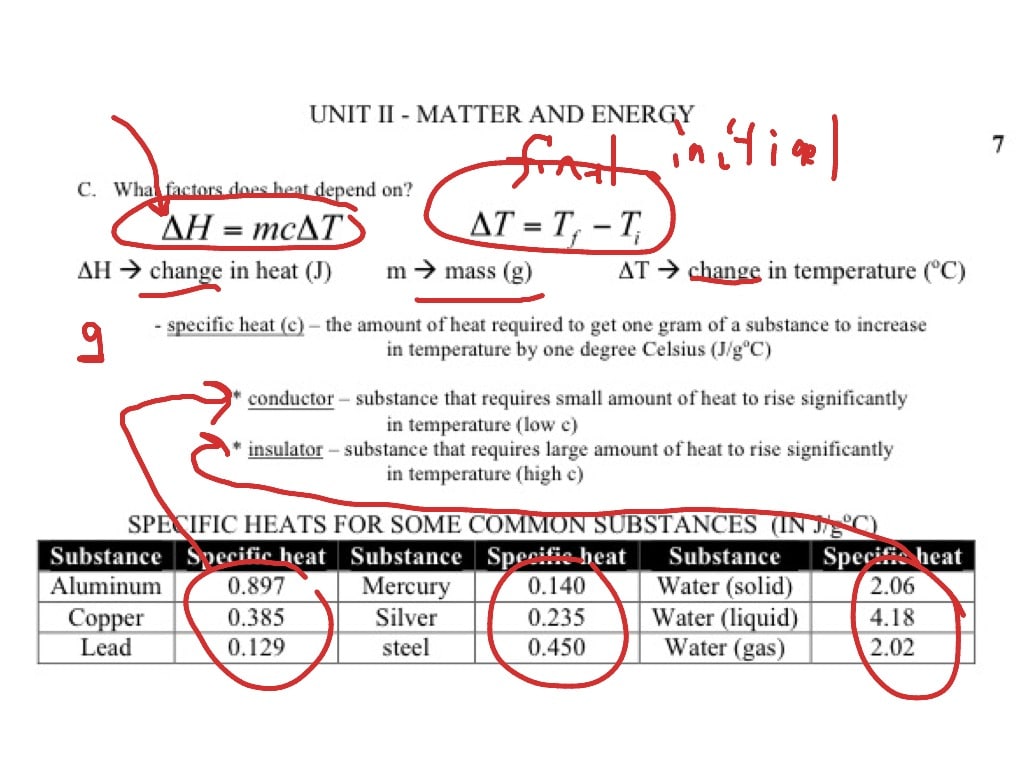
The quantity of heat that must be taken in or lost for one gram of material so that its temperature changes by one degree Celsius is referred to as specific heat.
The equation below shows the link between heat transferred and temperature change :
In this equation, m represents the substance’s mass whereas the value c represents the specific heat of the substance.
Water has one of the highest specific heat among common material substances at approximately 1 calorie/gram °C = 4.2 joule/gram °C.
What Is Heat Capacity
Heat capacity, Cp, is the amount of heat required to change the heat content of 1 mole of material by exactly 1°C.
In basic thermodynamics, the higher the temperature of a material, the more thermal energy it possesses. In addition, at a given temperature, the more of a given substance, the more total thermal energy the material will possess.
Image of Specific Heat of Water
On an atomic level, absorbed heat causes the atoms of a solid to vibrate, much as if they were bonded to one another through springs. As the temperature is raised, the energy of the vibrations increases. In a metal, this is the only motion possible. In a liquid or gas, absorbed heat causes the atoms in the molecule to vibrate, and the molecule to both rotate and move from place to place. Because there are more storage possibilities for energy in liquids and gases, their heat capacities are larger than in metals.
Also Check: What Does V Mean In Math
Relation Between Heat Capacities
Measuring the specific heat capacity at constant volume can be prohibitively difficult for liquids and solids. That is, small temperature changes typically require large pressures to maintain a liquid or solid at constant volume, implying that the containing vessel must be nearly rigid or at least very strong . Instead, it is easier to measure the heat capacity at constant pressure and solve for the heat capacity at constant volume using mathematical relationships derived from the basic thermodynamic laws.
The heat capacity ratio, or adiabatic index, is the ratio of the heat capacity at constant pressure to heat capacity at constant volume. It is sometimes also known as the isentropic expansion factor.
Ideal gas
For an ideal gas, evaluating the partial derivatives above according to the equation of state, where R is the gas constant, for an ideal gas
- P
V /c_} of the heat capacity at constant pressure to heat capacity at constant volume. It is sometimes also known as the isentropic expansion factor.
Key Takeaways: Specific Heat Capacity
- Specific heat capacity is the quantity of heat needed to raise the temperature per unit mass.
- Usually, it’s the heat in Joules needed to raise the temperature of 1 gram of sample 1 Kelvin or 1 degree Celsius.
- Water has an extremely high specific heat capacity, which makes it good for temperature regulation.
In SI units, specific heat capacity is the amount of heat in joules required to raise 1 gram of a substance 1 Kelvin. It may also be expressed as J/kg·K. Specific heat capacity may be reported in the units of calories per gram degree Celsius, too. Related values are molar heat capacity, expressed in J/mol·K, and volumetric heat capacity, given in J/m3·K.
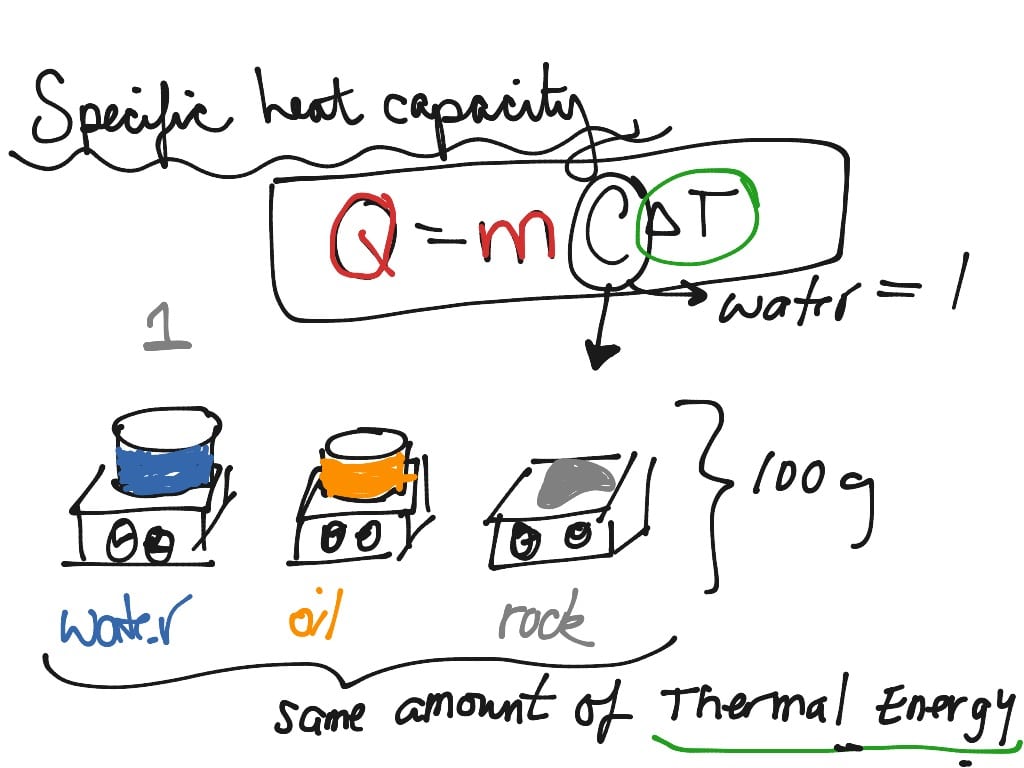
Heat capacity is defined as the ratio of the amount of energy transferred to a material and the change in temperature that is produced:
C = Q / T
where C is heat capacity, Q is energy , and T is the change in temperature . Alternatively, the equation may be written:
Q = CmT
Specific heat and heat capacity are related by mass:
C = m * S
Where C is heat capacity, m is mass of a material, and S is specific heat. Note that since specific heat is per unit mass, its value does not change, no matter the size of the sample. So, the specific heat of a gallon of water is the same as the specific heat of a drop of water.
Also Known As: specific heat, mass specific heat, thermal capacity
You May Like: How Does Geography Affect Our Lives
What Is The Specific Heat Capacity Of Water
Specific Heat Capacity of Water at normal temperature and pressure is roughly 4.2 J/g oC. This means that 1 gram of water requires 4.2 joules of energy to raise 1 degree Celsius. Water has a high specific heat capacity. The actual value of waters specific heat capacity is 4182 J/kg °C.
At normal temperature, water vapour has a higher specific heat capacity than most other materials. The specific heat capacity of water vapour at normal temperature and pressure is approximately 1.9 J/g°C.
Specific Heat Of Water
For liquid at room temperature and pressure, the value of specific heat capacity is approximately 4.2 J/g°C. This implies that it takes 4.2 joules of energy to raise 1 gram of water by 1 degree Celsius. This value for Cp is actually quite large. This is the specific heat of the water as a liquid or specific heat capacity of liquid water.
One calorie= 4.184 joules 1 joule= 1 kg2-2 = 0.239005736 calorie
The specific heat capacity of water vapour at room temperature is also higher than most other materials. For water vapour at room temperature and pressure, the value of specific heat capacity is approximately 1.9 J/g°C.
As with most liquids, the temperature of water increases as it absorbs heat and decreases as it releases heat. However, the temperature of liquid waterfalls & rises more slowly than most other liquids. We can say that water absorbs heat without an immediate rise in temperature. It also retains its temperature much longer than other substances.
We use this property of water in our body to maintain constant body temperature. If water had a lower Csp value, then there would a lot of cases of overheating and underheating.
Don’t Miss: Introduction To Functions Algebra 1 Worksheet
Relation Between Specific Heat Capacities
Starting from the fundamental thermodynamic relation one can show,
- c
- T . )=\int _^}}=\int _^}}}=\int _^}C\,}.}
The heat capacity must be zero at zero temperature in order for the above integral not to yield an infinite absolute entropy, thus violating the third law of thermodynamics. One of the strengths of the Debye model is that it predicts the proper mathematical form of the approach of heat capacity toward zero, as absolute zero temperature is approached.
What Is The Specific Heat Formula
While discussing the thermal properties of materials, specific heat is a concept that very few people discuss and are aware of it. We use the specific heat concept to understand how much heat has to be supplied to raise the temperature of an object by a degree in kelvin or celsius. The amount of heat required to raise the temperature of the substance is known as the specific heat. In other words, the specific heat is the amount of heat required to change the temperature of the material or substance under consideration.
In this article, we will discuss what is specific heat, what is the formula of specific heat along with a small derivation, and solve numerical problems.
Also Check: What Does Compass Rose Mean In Geography
Negative Molar Specific Heat Of A Gas
It is known that the specific heat of a gas is process dependent. So it must be theoretically possible to have a negative value for a gas according to the following equation :
$$C = \frac R + \frac R$$
where $C$ is molar specific heat and $$ is adiabatic exponent.
Supposing $$ is $\frac$ and $n$ is $\frac$, $C$ comes out to be negative. Is it practically possible and if so what would it signify? As you provide more heat to a gas in such a process, would it lose temperature?Please clarify.
- 1 PoutnikMay 2, 2019 at 17:01
- $\begingroup$But putting the values of monotomic gas and n as 4/3 seem to give a negative value.$\endgroup$ evamPUNditMay 2, 2019 at 17:06
- $\begingroup$While it is possible to add heat to gas and get the final temperature lower, one has to count done work and also possible change of C.$\endgroup$May 2, 2019 at 17:07
- 1May 2, 2019 at 17:10
- 5$\begingroup$It is a very bad idea to consider to consider heat capacity to be a function of process path . Doing this will drive you nuts in the future. In thermodynamics, we purposely define heat capacity as a function of either enthalpy or internal energy, both of with are functions of state . Problems like your polytropic gas example force you to think of heat capacity in the wrong way.$\endgroup$
What Is The Relationship Between The High Specific Heat Of Water And Its Chemical Bond
Water is made up of two hydrogen atoms connected by polar covalent bonds to one oxygen atom. When valence electrons are shared mutually by two atoms, it is referred to as a covalent bond.
Water is a polar molecule because its hydrogen and oxygen atoms share electrons unequally owing to electronegativity differences.
A polar molecule is one that has both a partially positive and a partially negative region.
Electronegativity is the tendency of an atom to attract and gain electrons.
Each hydrogen atom has a nucleus composed of a single positively charged proton and one negatively charged electron orbiting the nucleus. Each oxygen atom, on the other hand, has a nucleus composed of eight positively charged protons and eight uncharged neutrons, with eight negatively charged electrons orbiting the nucleus.
Because the oxygen atom has a higher electronegativity than the hydrogen atom, electrons are drawn to oxygen and repelled by hydrogen. During the formation of a water molecule, the ten electrons link up and form five orbitals, leaving behind two lone pairs. The two lone pairs associate themselves with the oxygen atom.
As a result, oxygen atoms have a partial negative charge, while hydrogen atoms have a partial positive charge. While the water molecule has no net charge, the hydrogen and oxygen atoms all have partial charges.
Also Check: What Is Locomotion In Biology
Specific Heat Capacity Definition
Specific heat capacity is the amount of heatenergy required to raise the temperature of a substance per unit of mass. The specific heat capacity of a material is a physical property. It is also an example of an extensive property since its value is proportional to the size of the system being examined.
What Is The Si Unit Of Specific Heat Capacity
Specific heat efficiency in SI units is the amount of heat required in joules to raise 1 gram of 1 Kelvin substance. It can be expressed as J / kg as well. · K. K. Specific heat capacity in calorie units per gram Celsius may be recorded.
Register with BYJUS to learn more about Heat Capacity and Specific Heat Capacity.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
You May Like: Holt Mcdougal Geometry Answers And Work