Ionic Bonds Vs Covalent Bonds
In this article, we discuss ionic bonding and covalent bonding, and compare ionic bonds vs covalent bonds. Well talk about what is an ionic bond, and what is a covalent bond. Well also give examples of both. Common table salt is an an example of common compound with ionic bonds. Ionic compounds are often solids, and form crystals.
Carbon dioxide, gas we breathe out of our lungs, is a compound with covalent bonds. But what is the difference between ionic vs covalent?
Bonding In Organic Chemistry
Ionic and covalent bonds are the two extremes of bonding. Polar covalent is the intermediate type of bonding between the two extremes. Some ionic bonds contain covalent characteristics and some covalent bonds are partially ionic. For example, most carbon-based compounds are covalently bonded but can also be partially ionic. Polarity is a measure of the separation of charge in a compound. A compound’s polarity is dependent on the symmetry of the compound and on differences in electronegativity between atoms. Polarity occurs when the electron pushing elements, found on the left side of the periodic table, exchanges electrons with the electron pulling elements, on the right side of the table. This creates a spectrum of polarity, with ionic at one extreme, covalent at another, and polar covalent in the middle.
Both of these bonds are important in organic chemistry. Ionic bonds are important because they allow the synthesis of specific organic compounds. Scientists can manipulate ionic properties and these interactions in order to form desired products. Covalent bonds are especially important since most carbon molecules interact primarily through covalent bonding. Covalent bonding allows molecules to share electrons with other molecules, creating long chains of compounds and allowing more complexity in life.
Practice Writing Correct Ionic Formulas
To predict and write correct chemical formulas, the key fundamental steps that are required, are knowing the charge states of the ions and using basic math to help you determine how many cations and anions are needed to reach a zero charge state, writing the chemical forumulas with the cation first followed by the anion, and writing the formula with the lowest ratio of cations and anions to create a net neutral compound.
Overall, ionic bonding occurs between a cation and an anion to form a compound that has an overall neutral net charge. Of note, ionic bonds usually occur between a metal and a nonmetal. This will help you recognize ionic compounds more easily, once we learn about covalent bonding .
So, say we want to write the correct chemical formula for a molecule that contains Fe3+ as the cation, and Cl as the anion. What is the correct ionic formula?
To begin this type of problem, I recommend drawing out a charge box or a charge table to help you keep track of the number of ions used, the charges of those ions, and the overall positive and negative charges on the molecule. Drawing out the electron dot symbols can also be helpful. Here is an example of a generic charge box
Lets try it out for our example of Fe3+ and Cl. First, lets fill in what we know about each element and its ionic state:
Don’t Miss: Definition Of Span Linear Algebra
Corrosionpedia Explains Chemical Instability
A chemical substance is said to be stable if it is not particularly reactive in the environment or during normal use, and retains its useful properties on the timescale of its expected usefulness. In this meaning, the material is said to be unstable if it can corrode, decompose, polymerize, burn or explode under the conditions of anticipated use or normal environmental conditions.
Unstable chemicals tend to undergo exothermic unimolecular decompositions. Variations in the structure of the related chemical species of this kind generally affects the energy of the transition states for these decompositions less than they affect the stability of the decomposing chemical species. Chemically unstable materials show less resistance to corrosion.
Instability refers to the susceptibility of the chemical to dangerous decomposition. Ethers, liquid paraffin and olefins can form unstable peroxides on exposure to air and light. Because these chemicals are packaged in an air atmosphere, peroxides can form even if their containers have not been opened. They are very unstable, flammable, and extremely sensitive to friction, impact, and heat.
Example : Composition Of Ions
An ion found in some compounds used as antiperspirants contains 13 protons and 10 electrons. What is its symbol?
Because the number of protons remains unchanged when an atom forms an ion, the atomic number of the element must be 13. Knowing this lets us use the periodic table to identify the element as Al . The Al atom has lost three electrons and thus has three more positive charges than it has electrons . This is the aluminum cation, \text^.
Check Your Learning
Read Also: Molecular Geometry Lab Answers
What Are Symbols In A Chemical Equation
4.2/5
| an alternative way of representing a substance in a gaseous state |
| indicates that the substance is in a solid state |
| an alternative way of representing a substance in a solid state |
APPENDIX 7: SYMBOLS IN CHEMICAL EQUATIONSChemical formula: indicates the number of each atom in a substance. Examples: H2 O2 H2O Chemical equation: indicates a chemical reaction with reactants and products. Example: hydrogen + oxygen
Secondly, what does the symbol triangle in a chemical equation mean? The symbol triangle in a chemical equation means. heat is supplied to the reaction.
Also question is, what are the symbols used in chemical reaction?
Use the common symbols, , , , , and appropriately when writing a chemical reaction.
What is a symbol equation?
A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and formulae, wherein the reactant entities are given on the left-hand side and the product entities on the right-hand side.
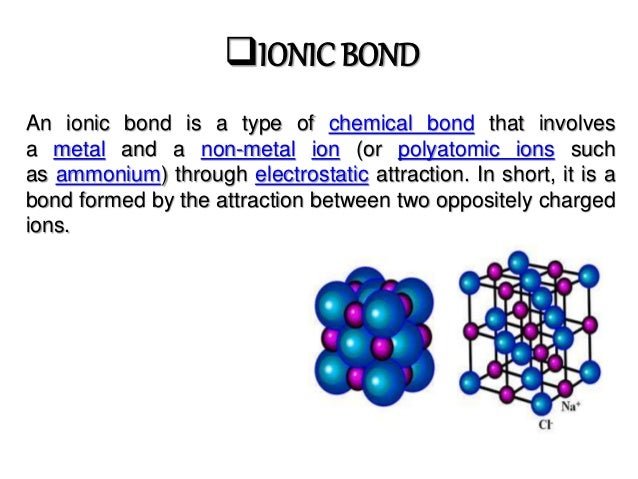
What Is An Ionic Bond
So what is an ionic bond? The definition of ionic bond, is a bond between atoms where electrons are transferred from one atom to another. We say mostly, because there is always some sharing of electrons between atoms, but in Ionic bonds, the sharing is very unequal. The less equal the sharing of the electrons, the more ionic character the bond has.
Ionic bonds occur between a metal and a non-metal. Unlike covalent bonds, ionic bonds transfer their valence electrons between atoms. In ionic bonding, the electronegativity difference between non-metals and metals exceeds 1.7. The metal atom transfers its electrons to the non-metal atom. Therefore, the metal atom becomes a positively charged cation and the non-metal atom becomes a negatively charged anion. Consequently, ionic bonds create two charged ions, the metal always donates its electron, and the non-metal always accepts the electron. An example of an ionic bond is the bond in sodium chloride, which is salt. Sodiums valence electron is transferred to the outer electron shell of chloride.
Molecules with ionic bonds form ionic compounds. Molecules with covalent bonds form covalent compounds. Covalent compounds often melt at lower temperatures, because their covalent bonds are easier to break. We hope you understand ionic vs covalent bonds and compounds a little better now.
You May Like: How Do You Find The Displacement
Arrhenius Acids And Bases
H+ and OH ions are the key players in acid-base chemistry, under the Arrhenius definitions for acids and bases. Arrhenius defined an acidas a compound that increases the concentration of hydrogen cations in aqueous solution. Many acids are simple compounds that release a hydrogen cation into solution when they dissolve and can be recognized as ionic compounds that contain H+ as the cation. Similarly, Arrhenius defined a baseas a compound that increases the concentration of hydroxide ions in aqueous solution. Many bases are ionic compounds that have the hydroxide ion as their anion, which is released when the base dissolves in water.
Arrhenius bases are named according to standard ionic nomenclature, with the strongest bases being the hydroxides of the alkali metals and the heavier alkaline earth metals. You will be expected to recognize strong bases.
HClO = hypochlorous acid
These are all distinguished from the binary chlorine-containing acid:
HCl = hydrochloric acid
Strong acids are ones that completely dissociate into their ionic forms in solution. The following table lists common strong acids that you will need to be familiar with.
Quiz Yourself: More Practice Naming Compounds
Ionic Bonding: Definition And Examples
Indeed, we see this type of ionic bond in many kinds of common materials, for example, sodium chloride or table salt. Whenever you have an alkali metal, like sodium, that meets up with a halogen, like fluorine or chlorine, they form a salt. The positive and negative ions attract each other, and so you get the bond. Now, because these bonds are between two ions, it is called the ionic bond.
And there are many examples from the periodic table. Sodium chloride is the most common example of these, but there are many others. For example, when you buy salt, youll often notice that its called iodized salt. Thats because we need a small amount of iodine in our diet to help the thyroid gland.
If we dont get it other ways, we can get it from table salt. Because in addition to having sodium chloride, table salt typically has about 1/100th of a percent of potassium iodine, another one of these alkali halides. And thats because potassium, in the periodic table, in the first column, bonds with iodine, the halogen, in the next to the last column.
Learn more about phase transformations and chemical reactions.
Also Check: Beth Thomas Brother Jonathan
Writing Formulas Of Ionic Compounds
Remember thePrime Directive in writing formulas:Ca2 ¹ CaOH2 !
Common Questions About Ionic Bonding In Chemistry
Q: What is ionic bonding?
In ionic bonding, electrons are transferred from one atom to another, resulting in the formation of positive and negative ions. Its the attraction created between positive and negative ions that creates a compound.
Q: What are some examples of ionic bonding?
There are many examples of ionic bonding in nature. Table salt is an excellent example of an ionic bond between sodium and chlorine. Silicon and oxygen also bond with a strong ionic bond to form quartz. It should be noted that the beach sand is made of quartz.
Q: What are the properties of ionic bonding?
Ionic bonding has three unique properties. These features include high resistance, electrical insulation, and transparency.
You May Like: How To Find Delta Math Answers With Inspect Element
What Are Ionic Compounds
Ionic compounds are compounds consisting of ions.
Two-element compounds are usually ionic when one element is a metal and the other is a non-metal. Examples include:
- sodium chloride: NaCl, with Na+ and Cl- ions
- lithium nitride: Li3N, with Li+ and N3- ions
- magnesium oxide: MgO, with Mg2+ and O2- ions
- calcium phosphide: Ca3P2, with Ca2+ and P3- ions
Ionic compounds can be more complicated than the two-element ones listed above. Examples of polyatomic ionic compounds include:
- sodium sulfate: Na2SO4, with Na+ and SO42- ions
- ammonium thiocyanate: NH4SCN, with NH4+ and SCN- ions
- potassium hydroxide: KOH, with K+ and OH- ions
- ammonium carbonate: 2CO3, with NH4+ and CO32- ions
Although they consist of positively and negatively charged ions, ionic compounds are electrically neutral, because the charges are always equal and opposite.
Corrosionpedia Explains Chemical Resistance
Chemical resistance is the ability of a material to withstand chemical attack. A material with high chemical resistance, therefore, has less chance of corrosion. Materials with high chemical resistance are generally regarded as corrosion-resistant materials due to their inertness to chemical attack.
A material with low chemical resistance generally results in swelling or softening of the material, hence the material loses serviceability. When testing a material for chemical resistance, important factors include:
- Temperature
- Exposure duration
- Mechanical load
In a chemical resistance test, alumina and silicon carbide possess higher levels of resistance to chemicals, whereas stainless steel, zirconia and silicon possess lower levels of resistance to chemicals. Stainless steel is soluble in hydrochloric acid, whereas zirconia and silicon nitride are soluble in hydrofluoric acid, thereby they exhibit higher levels of solubility and lower levels of chemical resistance.
Chemically resistant products are necessary in varied applications and environmental conditions. In industry, corrosive chemicals are not only used, but also water sometimes causes corrosion to many materials. Therefore, use of products with high chemical resistance in adverse environments provides benefits in increasing industries’ effectiveness and productivity while lowering the need for parts replacement. It ultimately makes the process more cost-effective.
Related Question
Read Also: Cpm Algebra 2 Chapter 1 Answers
Example : Predicting The Formula Of A Compound With A Polyatomic Anion
Baking powder contains calcium dihydrogen phosphate, an ionic compound composed of the ions Ca2+ and }_}_^. What is the formula of this compound?
The positive and negative charges must balance, and this ionic compound must be electrically neutral. Thus, we must have two negative charges to balance the 2+ charge of the calcium ion. This requires a ratio of one Ca2+ ion to two }_}_^ ions. We designate this by enclosing the formula for the dihydrogen phosphate ion in parentheses and adding a subscript 2. The formula is Ca2.
Check Your Learning
Predict the formula of the ionic compound formed between the lithium ion and the peroxide ion, }_^
Formation Of Ionic Bond
An ionic bond can be formed after two or more atoms loss or gain electrons to form an ion. Ionic bonds occur between metals, losing electrons, and nonmetals, gaining electrons. Ions with opposite charges will attract one another creating an ionic bond. Such bonds are stronger than hydrogen bonds, but similar in strength to covalent bonds.
Eric Stauffer, … Reta Newman, in, 2008
Recommended Reading: 6 Major Branches Of Chemistry
What Does Chemical Resistance Mean
Chemical resistance is the strength of a material to protect against chemical attack or solvent reaction. It is the opposite of chemical reactivity. It determines a materials resistivity to corrosive environments.
Chemical resistance normally describes to what extent a material can maintain its resistance to chemicals. A chemical resistance chart may be used to see the relative resistance of materials. Thermoplastics are suitable to use in many industrial process applications due to their high resistance to many chemicals.
Chemical resistance may also be known as chemical inertness.
What Are The 2 Parts Of An Ionic Compound
Ionic compounds are compounds made up of ions that form charged particles when an atom gains or loses electrons. A cation is an ion charged positively an anion is an ion charged negatively.
To learn more about ionic compounds, register with BYJUS and download our app.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
Read Also: Prince And Paris Jackson Biological Father
What Are Coefficients In Chemistry
Coefficients are the numbers placed before the reactants in a chemical equation so that the number of atoms in the products on the right side of the equation are equal to the number of atoms in the reactants on the left side. If a written chemical reaction were not balanced in this manner, there would be no information available regarding the relationship between the reactants and products. Also known as stoichiometric coefficients, these numerical values demonstrate that the number of atoms on either side of the equation are equal to each other.
The need for balanced chemical equations is dictated by the law of conservation of mass. The law states that the quantity of each element involved in a chemical reaction does not change. For example, this is an unbalanced equation: N2 + O2 > NO. The nitrogen atoms and oxygen atoms on the left-hand reactant side of the equation do not equal the number of atoms on the right-hand product side. Placing a coefficient of 2 before the product, however, will balance out the equation and is written as N2 + O2 > 2NO.
Example : Predicting The Type Of Bonding In Compounds
Predict whether the following compounds are ionic or molecular:
1. Ionic: KCl, MgCl2 Covalent: NCl3, ICl, PCl5, CCl4
3. covalent ionic, Ba2+, O2- ionic, }_^, }_^ ionic, Sr2+, }_}_^ covalent ionic, Na+, O2-
5. CaS 2SO4 AlBr3 Na2HPO4 Mg3 2
Don’t Miss: Im.kendall Hunt Answers
Example : Formation Of Ions
Magnesium and nitrogen react to form an ionic compound. Predict which forms an anion, which forms a cation, and the charges of each ion. Write the symbol for each ion and name them.
Magnesiums position in the periodic table tells us that it is a metal. Metals form positive ions . A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. The symbol for the ion is Mg2+, and it is called a magnesium ion.
Nitrogens position in the periodic table reveals that it is a nonmetal. Nonmetals form negative ions . A nitrogen atom must gain three electrons to have the same number of electrons as an atom of the following noble gas, neon. Thus, a nitrogen atom will form an anion with three more electrons than protons and a charge of 3. The symbol for the ion is N3, and it is called a nitride ion.
Check Your Learning
Aluminum and carbon react to form an ionic compound. Predict which forms an anion, which forms a cation, and the charges of each ion. Write the symbol for each ion and name them.
| Table 1. Common Polyatomic Ions | |
|---|---|
| Charge | |
| phosphate | }_^ |