What Are The Steps Of Evaporation
In the water cycle, there are four key phases. Evaporation, condensation, precipitation and collection are as they are. Lets look at the steps of both of these. Evaporation: This is where the suns heating induces water to rise into the air from seas, lakes, streams, glaciers and soils, and transform into water vapour .
What Is High Heat Of Vaporization
High Heat of Vaporization
The Heat of Vaporization is the amount of energy to convert 1g or a substance from a liquid to a gas. In order for water to evaporate, hydrogen bonds must be broken. Waters heat of vaporization is 540 cal/g. In order for water to evaporate, each gram must GAIN 540 calories .
Ever Wondered Why Water Is Stored In Earthen Pots
The science involved in this medieval practice is evaporation. Water is stored in earthen pots especially in summer to keep the water cool. Earthen pots are made up of clay particles and have pores in them. When water is poured into the pot, it gets evaporated from the pores by absorbing heat from the remaining water in the pot.
This causes the cooling of the remaining water in the pot due to heat loss. Hence, water is kept cool which makes it suitable for drinking during summers.
Read Also: What Is Edge In Geometry
Heat Of Vaporization Problem
This sample problem demonstrates how to calculate the amount of energy required to turn a sample of water into steam:
What is the heat in joules required to convert 25 grams of water into steam? What is the heat in calories?What you know: Heat of vaporization of water = 2257 J/g = 540 cal/g
Note: You won’t be expected to know enthalpy or heat values they will be given in a problem or can be looked up in a table.
What Is The Evaporation Process
The evaporation process is the transition from the liquid phase to the gas phase taking place below the boiling temperature at the given pressure. Also, and it happens on the surface and not bellow as in the vaporization process. Thus evaporation is a type of vaporization process of liquid into the gas state on its surface.
Recommended Reading: What Is Difference Threshold In Psychology
What Is The Heat Of Fusion And Vaporization Of Water
The heat of fusion for water at 0 °C is approximately 334 joules per gram, and the
heat of vaporization
heat of vaporization
energy transfer
latent heat, also called the heat of vaporization, is the amount of energy necessary to change a liquid to a vapour at constant temperature and pressure. The energy required to melt a solid to a liquid is called the heat of fusion, and the heat of sublimation is the energy
https://www.britannica.com
How Is The Heat Of Vaporization Of Carbon Dioxide Different From That Of Water
Explanation: Carbon dioxide is not a good comparison to water. Its molar mass is 44.009 g/mol , which is 2.4 times the molar mass of water. Also, at normal atmospheric pressure, carbon dioxide sublimes from the solid state directly to the gaseous state, so it has a heat of sublimation, not a heat of vaporization.
Don’t Miss: Geometry Dash Blast Processing Full Ver
What Is Vaporization In Chemistry
This is a question our experts keep getting from time to time. Now, we have got the complete detailed explanation and answer for everyone, who is interested!
Asked by: Mr. Denis Lindgren I
vaporization, conversion of a substance from the liquid or solid phase into the gaseous phase. If conditions allow the formation of vapour bubbles within a liquid, the vaporization process is called boiling.
How Does Evaporation Cause Cooling
- Evaporation causes cooling naturally. The underlying principle behind this is that in order to change its state, the matter must either gain or lose energy. In the case of change of phase from liquid to gas, molecules of matter require energy to overcome their potential energy by their kinetic energy. So, the liquid takes this energy from its surroundings.
- Generally, when energy transfer occurs, it results in an increase or decrease in the temperature of the substance, depending on whether the energy is being transferred from the substance to the surroundings or vice versa. However, there are exceptions to this rule.
- Although there is an increase in temperature of the substance until the boiling point is attained during evaporation, phase change results in no observable heat transfer.
- The molecules of the substance absorb heat energy continuously from the surroundings and thus cool the surroundings until they reach the boiling point, after which they start to break free from the liquid and turn into vapour. Since there is no change in temperature till the evaporation process is complete i.e. the entire liquid gets converted into vapour, the amount of energy required for this phase change is called the latent heat of vaporization, where the word latent means hidden, meaning this heat will not change the temperature reading on a thermometer.
Recommended Reading: Who Established The First Psychological Laboratory
How Does Vaporization Happen
Vaporization on Atomic Level
The temperature increase causes molecules to move quickly and this motion breaks the intermolecular bonds between the atoms. As these bonds are broken the molecules and atoms separate and spread out which causes them to vaporize or turn into a gas.
Vapor Pressure Of Electrolyte Solutions
Read Also: How To Study Chemistry For Class 12 Hsc
Solid To Gas Phase Transition
Applications Of Evaporative Cooling
Schematic Representation of Working of an Air Cooler
Don’t Miss: Who Is Known As The Father Of Nuclear Physics
Can Room Temperature Evaporate Water
The heat in that water results in some molecules transferring speedy enough to flee into the air that is evaporate. No further source of power is required for evaporation and the water does no longer wish to succeed in the boiling point to evaporate. As weve seen water will evaporate at room temperature.
What Is The Vaporization Process
This is a transitional phase of an element or compound from its solid phase or liquid phase to the corresponding gas phase. It also refers to the physical destruction of a substance with the effect of intense heat. Vaporization is the process of applying heat for the transition of some substance from a solid or liquid state to a gas state. It is must be noted that these changes in the state of matter from one to another are happening without a change in the chemical composition.
Also Check: How To Find Maximum Height Physics
Phase Transitions: Vaporization And Condensation
The physical form of a substance changes on changing its temperature. For example, raising the temperature of a liquid causes the liquid to vaporize . The process is called vaporizationa surface phenomenon. Vaporization occurs when the thermal motion of the molecules overcome the intermolecular forces, and the molecules escape into the gaseous state. When a liquid vaporizes in a closed container, gas molecules cannot escape. As these gas phase molecules move randomly about, they will occasionally collide with the surface of the condensed phase, and in some cases, these collisions will result in the molecules re-entering the condensed phase. The change from the gas phase to the liquid is called condensation.
Vaporization is an endothermic process. The cooling effect is evident after a swim or a shower. When the water on the skin evaporates, it removes heat from the skin and cools the skin. The energy change associated with the vaporization process is the enthalpy of vaporization, Hvap. For example, the vaporization of water at standard temperature is represented by:
The reverse of an endothermic process is exothermic. And so, the condensation of a gas releases heat:
Vaporization and condensation are opposing processes consequently, their enthalpy values are identical with opposite signs. While the enthalpy of vaporization is positive, the enthalpy of condensation is negative.
What Is Distinction Between Vaporization And Evaporation
Vaporization is outlined as the transitional segment of a compound or a component and it occurs all over the boiling or sublimation process. Evaporation is not anything but a kind of vaporization which most commonly happens at temperatures under the boiling level. These are one of the most variations between vaporization and evaporation.
Recommended Reading: Glencoe Geometry Chapter 10 Answers
Why Is Vaporization A Floor Phenomenon
Why is Evaporation a Surface Phenomenon? Evaporation is a floor phenomenon because right through evaporation the molecules with upper kinetic power fritter away into the air from the topmost layer of the liquid so it is a surface phenomenon. Therefore evaporation takes position from the surface but now not from the inside.
What Happens To Particles Before During And After Vaporization
If a liquid is heated the particles are given more energy and move faster and faster expanding the liquid. The most energetic particles at the surface escape from the surface of the liquid as a vapour as it gets warmer. Liquids evaporate faster as they heat up and more particles have enough energy to break away.
You May Like: What Is Visual Information Processing In Psychology
Difference Between Vaporization And Evaporation
Evaporation and Vaporization are the two commonly happening processes in our nature. These two processes are the true reasons why we need to water our garden in the summer season and also why the milk boils in the pot on the stove. Actually evaporation is a type of vaporization which takes place in our surroundings. So, evaporation is more common in comparison to other types of vaporization like boiling. Here we will discuss and explain the difference between vaporization and evaporation processes. Also, we make it clear that these two processes are not exactly the same, as many people tend to be confused considering both as same process. While these two appear to be words with similar meanings, but still there are many differences that exist. Actually, on a molecular level, both these processes differ from each other minutely. Both are occurring at different surfaces and temperatures.
Table of content
Factors Affecting The Rate Of Vaporization
You May Like: How Does Geography Help Us Plan For The Future
Why Is Heat Of Sublimation Greater Than Heat Of Vaporization
The force of attraction between solid particles is stronger than the force of attraction between liquid particles. Because of this, a higher amount of heat is needed to sublime the substance than evaporating it. As a result, the enthalpy of sublimation will always be larger than the enthalpy of vaporization.
Why Is The Latent Heat Of Vaporization Of Water So Much Greater Than The Latent Heat Of Melting
The energy required to completely separate the molecules, moving from liquid to gas, is much greater that if you were just to reduce their separation, solid to liquid. Hence the reason why the latent heat of vaporization is greater that the latent heat of fusion. So it really isnt a change in kinetic energy.
You May Like: What Is The Gravity Model Ap Human Geography
What Is Difference Between Vaporization And Evaporation
Vaporization is defined as the transitional phase of a compound or an element and it occurs during the boiling or sublimation process. Evaporation is nothing but a type of vaporization which mostly occurs at temperatures below the boiling point. These are some of the differences between vaporization and evaporation.
How Does Evaporation Occur At Room Temperature
You might be wondering how that can happen when the temperature is low. It turns out that all liquids can evaporate at room temperature and normal air pressure. Evaporation happens when atoms or molecules escape from the liquid and turn into a vapor. Not all of the molecules in a liquid have the same energy.
You May Like: What Is Theorem In Math
How Does Vaporization Occur
There exists two types of vaporization: evaporation and boiling. Evaporation is a surface phenomenon and only occurs on the phase boundary between the liquid and the gaseous phase. The surface atoms or molecules gains energy from surroundings and overcome the attractions of other molecules and get vaporized.
What Is The Main Difference Between Boiling And Vapourization
Stay tuned with BYJUS to learn interesting topics and for further queries please get in touch with the mentor support team at BYJUS.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
Also Check: How To Calculate Hybridisation In Chemistry
What Is An Example Of Vaporization
Examples of Liquid to Gas
Water to steam Water is vaporized when it is boiled on the stove to cook some pasta and much of it forms into a thick steam. Water evaporates Water evaporates from a puddle or a pool during a hot summers day.
High Heat Of Vaporization
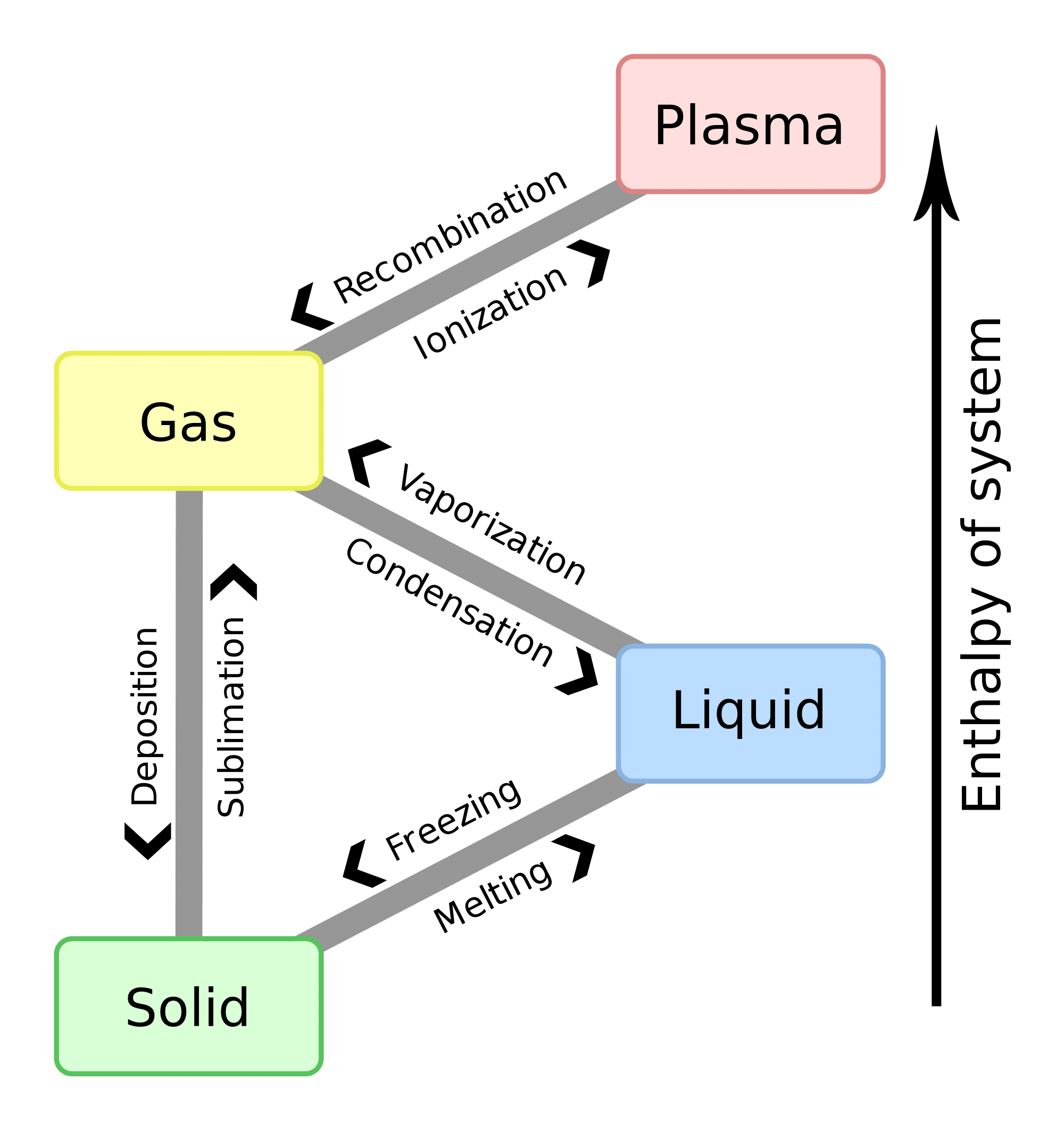
The high heat of vaporization is defined as the heat required to convert 1 gram of liquid into the gaseous state. As the temperature rises, the hydrogen bonds present in the liquid water start to break. Water has the highest known heat of vaporization. Whenever heat is provided to the water, molecules start getting dismissed and they vaporize. The surface molecules get cool and hence More kinetic energy is required to evaporate water and hence water has the highest heat of vaporization.
Whenever a liquid in a closed container is heated, the liquid which is getting converted into a gaseous state, through evaporation is unable to escape. This process continues until there are as many molecules as the liquid state. At this point, the vapor is said to be saturated vapor, and the pressure associated with it is saturated vapor pressure.
Also Check: What Does The Slash Mean In Math
What Is Vaporization
Vaporization can be defined as the process in which the liquid state changes into the vapour state. As a result of an increase in temperature, the kinetic energy of the molecules increases. Due to the increase in kinetic energy, the force of attraction between the molecules reduces. As a result, they escape into the surrounding in the form of vapours. This process involves the consumption of heat energy.