Easy Method To Determine Hybridization Of Atoms With Examples
Hybridization is a section of bonding in chemistry. In examinations, you may have some questions such as identifying hybridization of atoms and determine which hybridized orbitals are attached to make bonds. At the end of this tutorial, you will have the ability of determine the hybridization of atoms in a short time.
Hybridization Of Atoms With Electron Pairs Next To Double Or Triple Bonds
Ive mentioned above, that a double or a triple bond next to an electron pair matters. When you have an electron pair next to a p-orbital or a -bond, theres a resonance between those.
This is a very typical trick question on the exam, so you wanna keep this in mind. Its also important to remember that the electron pair has to be physically able to align with the p-orbital or a -bond for this to happen. So, the isolated electron pairs will still be sitting on the hybrid orbitals even when they are next to double bonds.
Spotting the isolated electron pairs can be a little tricky, so you may wanna do some practice to master this skill. There is a quick rule of thumb you can use. If you have an electron pair on the atom that already has a double bond, chances are, its going to be isolated. Its not a 100% foolproof trick, but it works for cyclic structures.
Are you ready to tackle some practice questions?
How To Determine The Hybridization Of A Molecular Compound
wikiHow is a wiki, similar to Wikipedia, which means that many of our articles are co-written by multiple authors. To create this article, volunteer authors worked to edit and improve it over time. This article has been viewed 49,226 times.
Determining the hybridization can be difficult. This wikiHow will help you determine the molecular geometry and the hybridization of the molecular compound. First you must draw the Lewis Structure, or determine the molecular geometry to help find the hybridization.
Read Also: What Is The Hardest Level In Geometry Dash
The Logic For Hybridisation Formula
Hybridisation for a molecule is given by:$$\frac$$Where,
If this expression is 2 then its $sp$ hybridised, 3 then $sp^2$ hybridised and so on.However, I am not able to understand why this works.
- 4Dec 20 ’15 at 17:01
- 4
Since you understand why it works for monovalent surrounding atoms, I will assume that we have a molecule $\ce B_}$ where $\ce$ is the central atom, $\ce$ represents the monovalent atoms which may or may not be identical, and $\ce$ represents not necessarily identical atoms which are polyvalent. First up, there are some assumptions to be made:
Find Hybridisation Of Carbon Compounds
- 92
DrDu said:May be you could describe what you consider to be the “usual” way?
- 6,195
- 845
This is certainly not the usual way to look at hybridization. I have never seen this before and in fact it seems to make not the least sense at all. Where did you find this number mysticism?
Pranav-Arora said:Why doesn’t it make sense?I think that’s the most easiest way to find the hybridisation. This is just a trick to find the hybridisation. Which way do you use to find hybridisation?I forgot to mention what’s the hybridisiation in the example.NF3 has sp3 hybridisation. 3 + 1= 4. 4 indicate sp3 hybridisation.
And why should this one lone pair which you calculate belong to N and not to one of the F?
DrDu said:On an elementary level it is not difficult to work out the number of bonds and lone pairs in a given molecule. Just make sure that every main group element has 8 electrons around at least in “normal” compounds eventually introducing some formal charges on the atoms. The hybridisation which is preferably to be used to describe a molecule depends not only on the number of electron pairs but also on the geometry, thats why you are having probably problems with carbon compounds. Finally, your hhybridization paterns 5 to 7 don’t appear at least in main group compounds, although this is still repeated in old or bad books. A still very readable introduction to the principles is the classic book by Linus Pauling, General chemistry.
Don’t Miss: What Does Co Stand For In Chemistry
Determining The Structure Of N2o Using Hybridization
I have learnt how to determine the structure of molecules where only the central atom is hybridised like $\ce$, $\ce$, $\ce$, but in $\ce$ it seems as though both nitrogen and oxygen have hybrid orbitals. How do I find the hybridization of oxygen and nitrogen in $\ce$ and finally determine its structure?
Research Effort: I watched some lectures on hybridization, but all of them included cases in which only the central atom had hybrid orbitals. I also read the topic in my book but this issue wasn’t addressed there too.
- 8$\begingroup$I don’t see how the hybridsation model could yield a structure prediction. In my understanding hybridisation can be used a post Lewis/VSEPR justification of a structure prediction. In that line you 1. draw a proper Lewis representation 2. apply VSEPR and 3. then explain it using hybridisation.$\endgroup$
Usually you would approach this problem from the other direction. First, find the most likely structure with the correct number of bonds, then deduce the hybridisation. This is also how it works in quantum chemistry: hybridisation is deduced from geometry, not the other way around.
If you try to write the structure of $\ce$ you should come up with two equally like structures these are mesomeric structures as given in the spoiler tag below.
$$\ce=\overset=O < -> N#\overset-\overset}$$
To do this, you will need to follow the steps mentioed by R_Berger in the comments. I will use $\ce$ as an example.
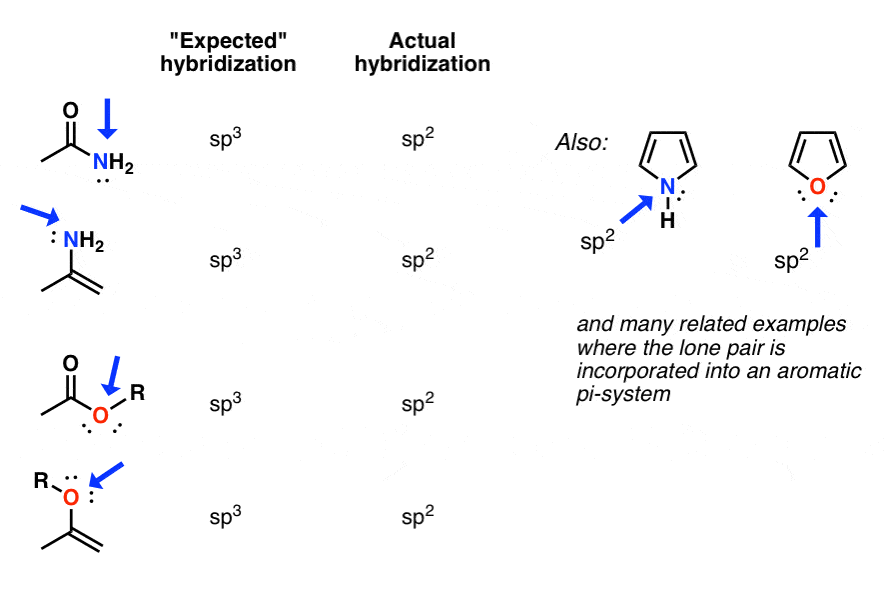
An Exception To The Steric Number Method
One exception with the steric number is, for example, the amides. The nitrogen atom here has steric number 4 and expected to sp3. However, because of the resonance delocalization of the lone pair, it interconverts from sp3 to sp2 as it is the only way of having the electrons in an aligned p orbital that can overlap and participate in resonance stabilization with the pi bond electrons of the C=O double bond.
In most cases, you wont need to worry about the exceptions if you go based on the Steric Number.
Practice
Determine the hybridization state of each carbon and heteroatom in the following compounds.
Hint: Remember to add any missing lone pairs of electrons where necessary.
Read Also: What Are The Four Stages Of Sleep In Psychology
Hybridisation Theory Vs Mo Theory
Hybridisation theory is an integral part of organic chemistry and in general discussed together with molecular orbital theory in advanced organic chemistry textbooks although for different reasons. One textbook notes that for drawing reaction mechanisms sometimes a classical bonding picture is needed with 2 atoms sharing two electrons . It also comments that predicting bond angles in methane with MO theory is not straightforward. Another textbook treats hybridisation theory when explaining bonding in alkenes and a third uses MO theory to explain bonding in hydrogen but hybridisation theory for methane.
Although the language and pictures arising from Hybridisation Theory, more widely known as Valence Bond Theory, remain widespread in synthetic organic chemistry, this qualitative analysis of bonding has been largely superseded by molecular orbital theory in other branches of chemistry. For example, inorganic chemistry texts have all but abandoned instruction of hybridisation, except as a historical footnote. One specific problem with hybridisation is that it incorrectly predicts the photoelectron spectra of many molecules, including such fundamental species such as methane and water. From a pedagogical perspective, hybridisation approach tends to over-emphasize localisation of bonding electrons and does not effectively embrace molecular symmetry as does MO Theory.
Some Simple Worked Examples Of The Hybridization Shortcut
sp3 hybridization: sum of attached atoms + lone pairs = 4
sp2 hybridization: sum of attached atoms + lone pairs = 3
sp hybridization: sum of attached atoms + lone pairs = 2
Where it can start to get slightly tricky is in dealing with line diagrams containing implicit hydrogens and lone pairs. Chemists like time-saving shortcuts just as much as anybody else, and learning to quickly interpret line diagrams is as fundamental to organic chemistry as learning the alphabet is to written English.
Remember:
- Just because lone pairs arent drawn in on oxygen, nitrogen, and fluorine doesnt mean theyre not there.
- Assume a full octet for C, N, O, and F with the following one exception: a positive charge on carbon indicates that there are only six electrons around it. .
You May Like: Is Chemistry Or Physics Harder
Bonus Section: An Answer To A Question A Few People Might Be Asking
Alkynes are stronger acids than typical alkanes .
For example, alkynes are readily deprotonated by strong bases such as NaNH2, whereas alkanes are not:
Why? Because the CH bond in an alkyne has more s-character, and the resulting lone pair on carbon is held more tightly to the nucleus, rendering the conjugate base more stable.
Oh dear. There seems to be an angry mob approaching.
WAIT! You just said that alkyne CH bonds are stronger than alkane CH bonds because they have more s-character and now you are saying that they are easier to break because they have more s-character.
Shouldnt that make alkynes less acidic because the CH bonds have more s-character?
Put away the pitchforks! There is a perfectly logical explanation for this!
When Summation Of Number Of Sigma Bonds And Number Of Lone Pairs Around An Atom Equals To The 2 Or 3 Or 4
Summation of number of sigma bonds and number of lone pairs around an atom equals to the 2 or 3 or 4, only s and p orbitals have contributed for hybridization. Then our equation is simplified as below.
- Summation of number of sigma bonds and number of lone pairs around an atom = x + y
- Summation of number of sigma bonds and number of lone pairs around an atom = 1 + y
Therefore y value can be found by subtracting 1 from summation of number of sigma bonds and number of lone pairs.
y = summation of number of sigma bonds and number of lone pairs – 1
You May Like: How To Prepare Geography For Prelims
Key Principle On Orbital Hybridization And Bond Strengths: The Greater The S
Note that the trend for bond strengths, above, is sp > sp2> sp3
In other words, the more s-character on carbon, the stronger the bond.
Lets try this again. What about these three CC bonds?
Hopefully you ranked them A > B > C .
All else being equal: an sp-sp3 bond is stronger than an sp2-sp3 bond, which in turn is stronger than an sp3-sp3 bond.
Why?
Important Points To Remember

- In the formation of C2H2, the carbon atom needs extra electrons to form 4 bonds with hydrogen and other carbon atoms. As a result, one 2s2 pair is moved to the empty 2pz orbital.
- The 2s orbital in each carbon hybridizes with one of the 2p orbitals and forms two sp hybrid orbitals.
- Ethyne has a triple bond between the two carbon atoms.
Also Check: What Is Radiation In Chemistry
Homolytic Versus Heterolytic Bond Cleavage
Whats the source of the confusion here?
Lets follow the electrons.
Ultimately the resolution to this dilemma is recognizing the difference between homolytic cleavage and heterolytic cleavage .
Lets look at these two processes.
In homolytic cleavage of a CH bond, an electron is completely removed from the vicinity of the carbon and placed on hydrogen. Because of the greater s-character of the bond, it is more difficult to remove an electron from an sp-hybridized carbon than from an sp3 hybridized carbon. As we said above, thats why the bond-dissociation energies for alkyne CH bonds are higher than for alkane CH bonds.
In an acid-base reaction, the CH bond also breaks, but it breaks in such a way that the pair of electrons stays on the carbon atom. Since the bond breaks in a way that leads to an uneven distribution of electrons, it is called heterolytic bond cleavage .
Lets say that again: in an acid base reaction, the pair of electrons stays on carbon, resulting in a negatively-charged carbon atom .
Acidity, as measured by pKa, is a measure of the equilibrium between an acid and its conjugate base. The more that the equilibrium favours the conjugate base, the lower the pKa and the stronger the acid. In other words:
Any factor which makes the conjugate base more stable, increases acidity.
What lone pair will be more stable?
The lone pair held more tightly to the nucleus that is, the sp-orbital.
How To Find Hybridization Of Central Atom & Shape Of Molecule
Many students face problems with finding the hybridization of given atom in a compound and the shape of molecule. Nevertheless, it is very easy to determine the state of hybridization and geometry if we know the number of sigma bonds and lone pairs on the given atom. On this page, I am going to explain you how to determine them in 5 easy steps.
If you are not sure …..What is Hybridization in chemistry?….Watch the following video
Recommended Reading: What Does Pt Stand For In Math
What Orbitals Are Involved In The Tetrahedral Arrangement Of Methane
In the last post on the structure of methane we asked how we know that methane is tetrahedral.
Based on the orbitals of carbon we might have naively expected three of the C-H bonds to line up along the x, y, and z axes, respectively, and have the other one at some arbitrary angle
But then, as so often happens in science, our beautiful intuitive hypothesis was destroyed by some annoying experimental facts:
- methane has no measurable dipole moment
- the crystal structure of diamond is tetrahedral, with identical bond lengths and angles between carbons of 109.5 degrees. This isnt what wed expect if we were dealing with bonds between pure 2s and 2p orbitals!
In retrospect, the geometry makes sense. It happens that 109.5 degrees is the orientation that maximizes the distance between each of the four bonding pairs, and thus minimizes their repulsive interactions. In other words, the geometry is a direct consequence of opposite charges attract, like charges repel.
But how do we describe the orbitals that are used to give that bond angle?
This is a real chin-scratcher. They cant be pure 2s orbitals . And they cant be pure p orbitals, since the p orbitals are aligned at 90° to each other.
So what the heck kind of orbitals are they?
Hybridisation And Molecule Shape
Hybridisation helps to explain molecule shape, since the angles between bonds are approximately equal to the angles between hybrid orbitals. This is in contrast to valence shell electron-pair repulsion theory, which can be used to predict molecular geometry based on empirical rules rather than on valence-bond or orbital theories.
Don’t Miss: What Does Both Mean In Math
Examples Of Sp3d Hybridization
In sp3d hybridized orbitals, s orbital, three p orbitals and one d orbital are hybridized. When atom has sp3d hybridization, summation of number of sigma bonds and number of lone pairs around that atom should be 5.
Because all p orbitals are hybridized, y = 3. Therefore we can find the z from our equation.
- Summation of number of sigma bonds and number of lone pairs around an atom = x + y + z = 5
- 1 + 3 + z = 5
- z = 1
How Do I Figure Out The Hybridization Of A Particular Atom In A Molecule
I’m learning how to apply the VSEPR theory to Lewis structures and in my homework, I’m being asked to provide the hybridization of the central atom in each Lewis structure I’ve drawn.
I’ve drawn out the Lewis structure for all the required compounds and figured out the arrangements of the electron regions, and figured out the shape of each molecule. I’m being asked to figure out the hybridization of the central atom of various molecules.
I found a sample question with all the answers filled out:$\ce$
It is $\mathrm$ hybridized.
Where does this come from? I understand how to figure out the standard orbitals for an atom, but I’m lost with hybridization.
My textbook uses $\ce$ as an example.Carbon has $\mathrm$, but in this molecule, it has four $\mathrm$. I understand the purpose of four , but where did the “3” in $\mathrm$ come from?
How would I figure out something more complicated like $\ce$?
If you can assign the total electron geometry on the central atom using VSEPR, then you can always automatically assign hybridization. Hybridization was invented to make quantum mechanical bonding theories work better with known empirical geometries. If you know one, then you always know the other.
I assume you haven’t learned any of the geometries above steric number 6 , but they each correspond to a specific hybridization also.
$\ce$
For $\ce$
Also Check: Which Theories Of Motivation Has Biological Orientation
Add Number Of Sigma Bonds And Number Of Lone Pairs
When you add sigma bonds and lone pairs, most occasions you will get values like 1, 2, 3, 4, 5, 6. Lets take water molecule as an example.
In water molecule, there are two sigma bonds and two lone pairs around oxygen atom. Therfore, summation of number of sigma bonds and number of lone pairs around oxygen atom is 4.
According to the summation of number of sigma bonds and number of lone pairs around an atom, hybridization can be given as below.
- 1: not hybridized, only s orbital participates to the bonding
- 2: sp
- 5: sp3d
- 6: sp3d2
We can present a mathematical equation to find hybridization of atoms. Generally, we can represent the hybridization as sxpydz
Summation of number of sigma bonds and number of lone pairs around an atom = x + y + z
Because , there is only one s orbital in a period, always x = 1. Otherwise, we can say, for hybridization only one s orbital is contributed.