Simulating The Effect Of A Random Compartmentalization
To examine the overall impact of compartmentalization on inhibition, a number of random compartments were created. First, for each metabolite m and enzyme e of the inhibition network , the number of compartments n in which m and e are allowed to participate was randomly selected following a power-law distribution a*ba/x). Then, m and e was placed in n random compartments. Finally compared to the total 5,989 interactions of the inhibition network , the total number of inhibitory interaction removed due to rc compartments was counted. The entire process was repeated for 50 times.
What Is A Inhibition In Biology
inhibition, in enzymology, a phenomenon in which a compound, called an inhibitor, in most cases similar in structure to the substance upon which an enzyme acts to form a product, interacts with the enzyme so that the resulting complex either cannot undergo the usual reaction or cannot form the usual product …
How To Measure The Zone Of Inhibition
The zone of inhibition is measured using a ruler, a pair of calipers, or with the help of a template. Its size is measured in millimeters and usually rounded off to the closest millimeter. The diameter of the disk is also included.
These measurements are done by the naked eye without the help of any instrument. The measurement of the diameter is made from the back of the plate. These plates are held against a dark background.
These plates should be viewed directly from above in order to minimize the errors while measurement.
In case of organisms showing swarming motility, ignore the veil of the organisms that might swarm into the zone of inhibition.
In case there is no zone of inhibition around the disk, consider the zone of inhibition zero.
On blood agar, the zone of inhibition is measured from the upper surface of the agar and is done by removing the lid of the Petri plate.
Also Check: How Is Sociology Different From Psychology
What Is Inhibitory Control Task
Inhibitory control is broadly conceptualized as the ability to suppress or countermand a thought, action, or feeling. Many investigators study inhibitory control using carefully designed tasks like the stop-signal task, or the go/no-go task, that measure an individual’s ability to suppress a prepotent motor response.
Interactions Caused By Enzyme Inhibition
As with induction, interactions caused by enzyme inhibition are hard to anticipate from first principles. If in doubt about the possibility of an interaction, it is best to look it up or at the Indiana University Department of Medicine website cited previously .
Enzyme inhibition, particularly of CYP enzymes, slows the metabolism and hence increases the action of other drugs inactivated by the enzyme. Such effects can be clinically important and are major considerations in the treatment of patients with HIV infection with combination therapy, because several protease inhibitors are potent CYP inhibitors . Other examples of drugs that are enzyme inhibitors are shown inTable 10.5. To make life even more difficult, several inhibitors of drug metabolism influence the metabolism of different stereoisomers selectively. Examples of drugs that inhibit the metabolism of the active and less active isomers of warfarin in this way are shown inTable 10.6.
There are also examples of drugs that inhibit the metabolism of other drugs, even though enzyme inhibition is not the main mechanism of action of the offending agents. Thus, glucocorticosteroids andcimetidine potentiate a range of drugs, including some antidepressant and cytotoxic drugs.
A. Aloway, … Z.H. Song, in, 2017
Also Check: How To Differentiate In Physics
Metabolite Structural Similarity Calculations
To examine similarity between an inhibitor and substrates, a list of all substrates of the enzyme was extracted from the network reconstruction17. Then, for each inhibitor of this enzyme, the pairwise similarity to each of these enzyme metabolites was calculated, and only the best match considered as the similarity value. Further, to calculate similarity between inhibitor and any random compound, a list of random compounds of equal number as the enzymes metabolites was selected randomly from the list of all inhibitory metabolites. This process was repeated 50 times for both inhibitors and random metabolites. For example, a metabolite is an inhibitor for 5 enzymes , that have 2, 3, 3, 2, and 4 substrates each respectively, constituting in total a list of 14 substrates connected to the inhibitor. First, the similarity between the inhibitor and all 14 substrate metabolites is calculated. Then, 14 metabolites are randomly selected from the inhibitor network and their structural similarity compared with the inhibitor mapped to the enzyme. This random sampling is repeated 50 times, and eventually, 50 times the similarity score of the most similar random compound is plotted against the similarity score of the most similar enzyme metabolite.
Metabolite And Enzyme Essentiality
For every inhibitor, a list of genes directly involved in its metabolism was extracted from the reconstruction17. If any gene in the list is essential according to cell-specific essential genes61, then the metabolite was considered essential. Following the similar strategy for enzymes, a list of all genes associated with an EC number was used. If any gene in the list is essential according to cell-specific essential genes61, the enzyme was considered essential.
You May Like: What Does Hdi Mean In Geography
Metabolic Network Neighbours Are Most Likely Inhibitors
Metabolites which are metabolically derived from each other are structurally more likely similar as compared to more distal metabolites that underwent more enzymatic conversions . Indeed, structural similarity clusters strongly reflected topological distances within the inhibition network . Structural analogues are hence typically found within the close metabolic neighbourhood . Consistently, we find that the closer the metabolite is topologically to an enzyme, the more likely it is an inhibitor . As a consequence, inhibitors are enriched to occur within metabolic pathways . Metabolic feedback and feed-forward loops emerge hence most likely within metabolic pathways for structural reasons.
Inhibitors emerge in the metabolic neighbourhood of enzymes and are often the essential and most central metabolites.
Iii On The Basis Of Whether The Inhibition Is Reversible Or Irreversible
1. Reversible inhibition:
- The enzyme inhibition in which the enzymatic activity can be regained after removal of inhibitors.
- Types of reversible inhibition:
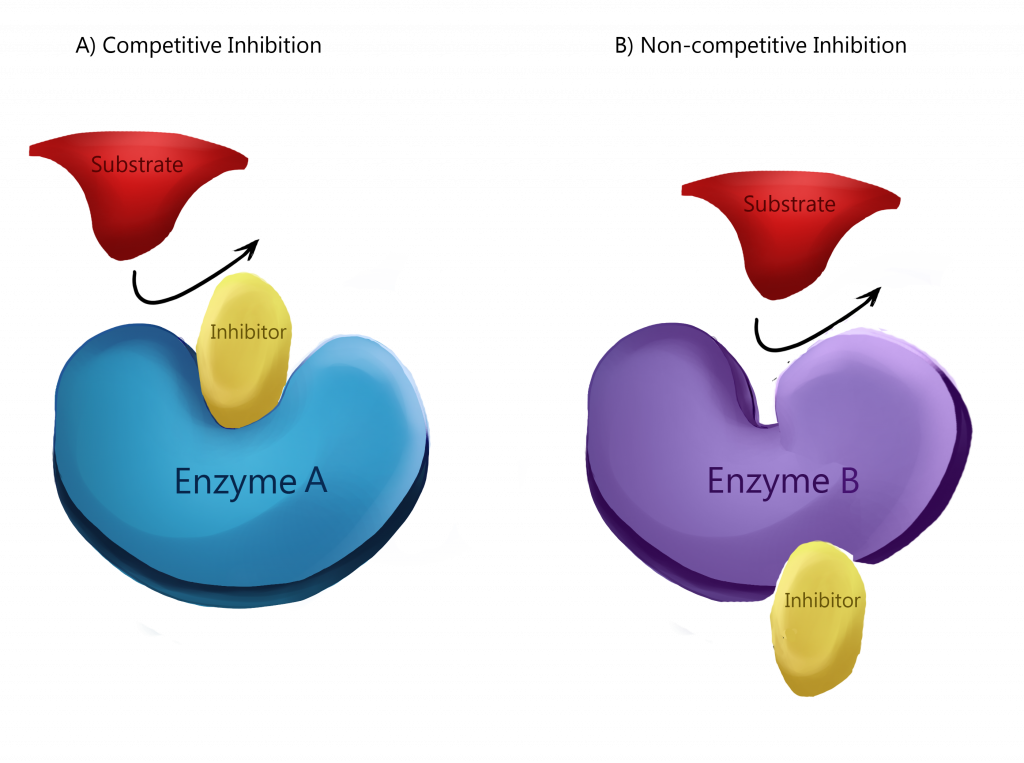
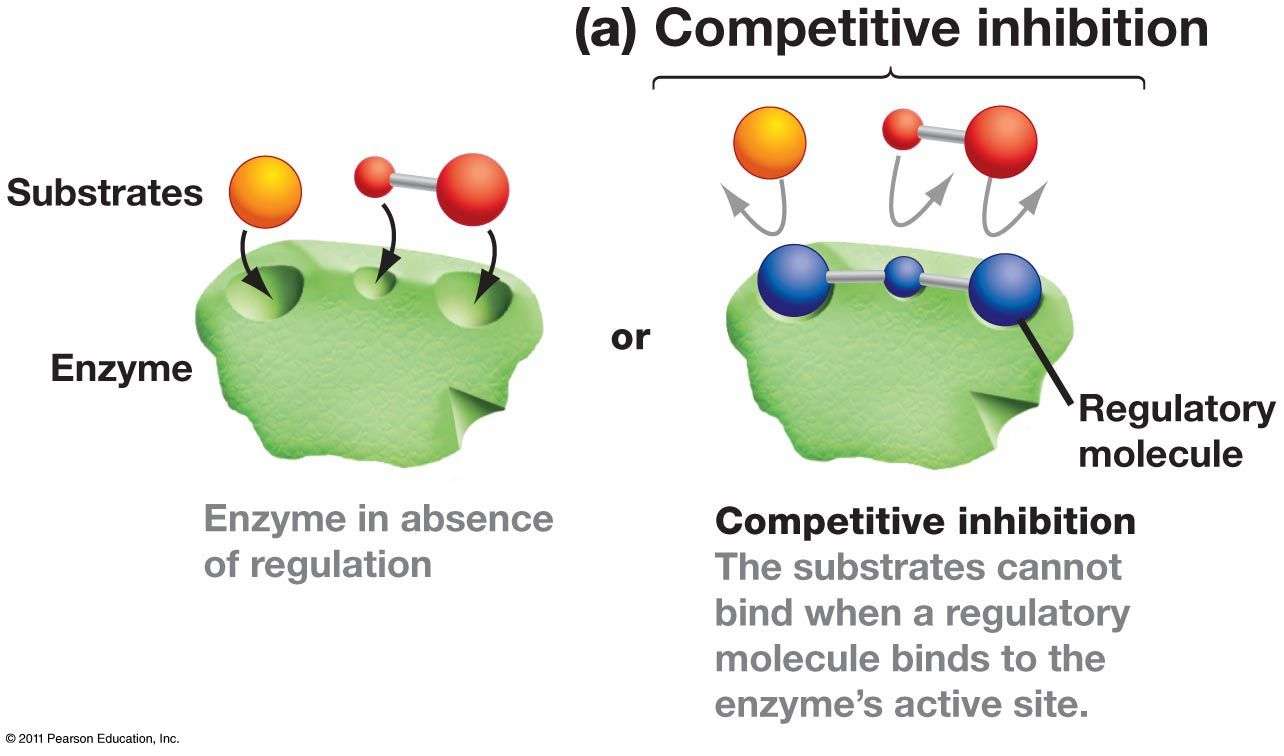
- i). Competitive inhibition
- Competitive inhibitors are substrate analog that bind to substrate binding site of enzyme i.e. active site so competition occurs between inhibitor and substrate for binding to enzyme.
- This type of inhibitor is overcome by increasing the concentration of substrate.
- The kinetics of reaction is Vmax remains same and Km increases.
- In this reaction, initially inhibitor binds to enzyme but with increase in concentration of substrate causes release of inhibitor.
- Then, substrate bind enzymes so that the Vmax remains same while Km increases.
- Example:
- Succinate dehydrogenase convert succinate to fumarate.Succinate succinate dehydrogenase> Fumarate + NADH +H+
- Malate is competitive inhibitor of succinate due to structural analogy.
- Malate + NAD+succinate dehydrogenase> Oxaloacetate
- Sulphonamide is competitive inhibitor of PABA during tetrahydrofolate synthesis.
Recommended Reading: Holt Mcdougal Algebra 1 Answer Key
Sensitivity Analysis Of Parameter Values
The presence of a number of parameters in our mathematical framework warranted a sensitivity analysis as to how the assigned parameter values affected the final computational results. We used two different metrics to ascertain parameter sensitivity. In the first analysis, we gauged the variation in the results by separately setting each one of the parameter values to reasonable lower and upper bounds , in this case, ± 50% of the chosen parameter values. In the second analysis, we computed the sensitivity coefficient for each of the parameters. This coefficient provides a measure of the dependency between the computed results and the corresponding parameter. If OD represents the cell concentration expressed as optical density under 600-nm-wavelength light and p represents the parameter analyzed for sensitivity, the sensitivity coefficient is defined as follows :
Other observables, different from OD, can be substituted for in Eq. . To numerically calculate the sensitivity coefficient for a parameter p, we started from p = +0.5p and repeated the process by reducing p and calculating the sensitivity coefficient until converged, that is, until successive values of p yielded the same . We then repeated the process starting from p = -0.5p until convergence. In the calculation performed here, both processes converged to the same numerical value.
Competitive Inhibition Dominates Metabolism
The biochemical literature distinguishes three major inhibitory mechanisms: competitive, noncompetitive and uncompetitive . Of these, competitive inhibition implies indeed a structural relationship between inhibitor and substrate23,24. We questioned which mechanisms would dominate. We manually curated the data set to create a gold standard of single-experiment determined reaction mechanisms. Competitive inhibition accounted for 74.6% of the gold-standard mechanisms, noncompetitive for 18.6% and uncompetitive inhibition for merely 6.7% . We selected three representative examples TPI, aconitase and PK, for which crystallographic structures both for the enzymes bound to substrate and competitive inhibitor are available5,25,26,27,28,29. In all three cases, the structures revealed striking structural similarity with a metabolic substrate .
Enzyme inhibition across the metabolic landscape is driven by structural similarity between metabolites.
Next, we used computational structure prediction to enumerate similarities between substrates and inhibitors. Pairwise compound comparisons with fingerprint similarity , drug molecule similarity derived by molecular fingerprints , maximum common substructure as well as pairwise compound comparisons with atom pairs , all revealed that the typical competitive inhibitor possesses significant similarity to at least one enzymatic substrate in the gold-standard data set .
Also Check: How Did Geography Affect Where Rome Was Located
Neural Crestneural Crest Interactions Promote Collective Guidance
By controlling cell polarity, CIL has a direct influence on the chemotactic abilities of the cells . Groups of Xenopus NCC are well guided by gradients of Sdf1. Cells polarize according to their cellcell contacts and CIL-dependent local inhibition of cell protrusions. Cxcr4 signaling promotes Rac1 activity, and thus protrusions facing high concentration of Sdf1 are stabilized . This favors migration toward the source of Sdf1. By contrast, single NCC poorly chemotax . If single cells are cultured at high cell density, allowing frequent collisions, individual NCC respond efficiently to chemotactic signals. Blocking CIL by affecting N-cadherin inhibits this collective chemotaxis . Therefore, the interplay between CIL and chemotaxis can explain the directional migration of NCC toward region of Sdf1 expression. N-cadherin and Sdf1 signaling are both required for NC cell migration in fish, but the possible connection between the two has not been assessed in this species .
Andrew C. Hedman, David B. Sacks, in, 2021
Components Of The Medium
Certain components of the medium like thymine, sulfonamide, or thymidine may inhibit the activity of antibiotics like trimethoprim, and may yield false results. For this reason, the Mueller-Hinton agar is the medium prescribed. This medium has a low thymine, sulfonamide, and thymidine content. This medium also contains starchit acts as a carbon source for the bacterium, and also absorbs toxic metabolites that may be produced by the organism.
Note: Usually, more than one antibiotic can be used on a plate. If, however, the zones of inhibition of two antibiotics merge, the readings should not be considered, and the experiment should be repeated.
You May Like: What Are Stressors In Psychology
Function Of Feedback Inhibition
Feedback inhibition allows the body to avoid many potentially dangerous situations, including:
- Waste. Without feedback inhibition, energy or raw materials that could be used for important cellular functions might be wasted on unnecessary ones.
- Prevents depletion. Without feedback inhibition, raw materials and energy might be depleted by biochemical processes that dont stop, even when their end product is not needed. A good example of this is the production of ATP from glucose. The enzymes that produce ATP from glucose are subject to feedback inhibition by ATP. This saves glucose by preventing its unnecessary breakdown when the cell has plenty of ATP.
- Prevents dangerous build-up. The end products of some biochemical pathways can actually be dangerous in high concentrations. Cholesterol is an excellent example of something our body can make that is good in small quantities but dangerous in large quantities.
- Maintain homeostasis. An essential function of life is the ability to maintain constant internal circumstances in the face of changing environmental circumstances. Some chemical messengers that are involved in maintaining homeostasis are regulated through feedback regulation.
Examples Of Allosteric Inhibition
An example of an allosteric inhibitor is ATP in cellular respiration. This metabolic process operates as a feedback loop. In this loop, downstream products control the speed of upstream reactions.
One enzyme involved in glycolysis is phosphofructokinase. It converts ADP to ATP. When there is too much ATP in the system, the ATP serves as an allosteric inhibitor. It binds to phosphofructokinase to slow down the conversion of ADP. In this way, ATP is preventing the unnecessary production of itself. There is no need to produce more ATP when there are already adequate amounts.
One example of an important drug that takes on the role of an allosteric inhibitor is the antibiotic penicillin. By helping the body kill off harmful bacteria, penicillin has saved millions of lives.
Harmful bacteria rely on the enzyme DD-transpeptidase to create strong, mesh-like cell walls. To counteract this process, penicillin binds to this enzyme. By acting as an inhibitor, penicillin prevents bacteria from building strong cell walls. With a weak wall, the surrounding fluids of the bacteria cell can then push itself in through osmosis. The cell will then burst and die.
Don’t Miss: How To Get The Distance In Physics
Contact Inhibition Nf2 And Apc
When cancer cells are grown in the laboratory, their proliferation fails to be inhibited when they come in contact with each other. This is in sharp contrast to nontransformed cells, which stop proliferating once they form confluent monolayers. The mechanisms that govern contact inhibition are only now being discovered. Cellcell contacts in many tissues are mediated by homodimeric interactions between transmembrane proteins calledcadherins. E-cadherin mediates cellcell contact in epithelial layers. Two mechanisms have been proposed to explain how E-cadherin maintains contact inhibition:
-
One mechanism is mediated by the tumor suppressor geneNF2. Its product, neurofibromin-2, more commonly calledmerlin, acts downstream of E-cadherin in a signling pathway that helps fo maintain contact inhibition. Homozygous loss ofNF2 is known to cause certain neural tumors, and germ line mutations inNF2 are associated with a tumor-prone hereditary condition calledneurofibromatosis type 2.
-
A second mechanism by which E-cadherin may regulate contact inhibition involves its ability to bind -catenin, another signaling protein. -catenin is a key component of the WNT signaling pathway , which has broad but as of yet incompletely understood roles in regulating the morphology and organization of epithelial cells lining structures such as the gut.
Katalin Szaszi, Yasaman Amoozadeh, in, 2014
Lock And Key: Substrate Binds To Enzyme At The Active Site
Metabolic processes consist of a series of chemical reactions that produce end products. The key drivers of metabolic processes are enzymes. Enzymes are specific proteins that catalyze reactions. These enzymes speed up important chemical reactions in cells by reducing the amount of energy that is required.
First, an enzyme binds to a substrate. This reaction then creates a product. The product can then serve as a subsequent substrate for a different enzyme at the next metabolic step. Finally, there is a chain of reactions that occur until a final product is created at the end.
One important point is that the binding of an enzyme and its substrate is very specific. The enzyme can be compared to a lock and the substrate can be compared to a key. Certain substrates can only bind to certain enzymes. They bind at a location on the enzyme called the active site.
The wrong key will not fit the specific lock on an enzyme. If the substrate cannot fit into an active site, the enzyme cannot catalyze a reaction. Even if the substrate is the correct substrate for an enzyme, an allosteric inhibitor can prevent the enzyme from having the correct shape or conformation.
Read Also: What Does Mass Mean In Math
What Does Zone Of Inhibition Mean And How To Measure It
Zone of inhibition is found with the help of disk diffusion method. This BiologyWise post gives you the definition as well as information regarding different parameters that may affect the zone of inhibition.
Like it? Share it!
Zone of inhibition is found with the help of disk diffusion method. This BiologyWise post gives you the definition as well as information regarding different parameters that may affect the zone of inhibition.
Applications Of Enzyme Inhibition
In the pharmaceutical and medical industries, enzyme inhibitors are essential. Pharmacologists benefit from a basic grasp of inhibitor activity during the development of new therapeutic medicines.
Insecticides such as malathion, herbicides such as glyphosate, and disinfectants such as triclosan are all examples of artificial inhibitors. Other synthetic enzyme inhibitors inhibit acetylcholinesterase, an enzyme that breaks down acetylcholine, and are utilised in chemical warfare as nerve agents.
Also Check: What Is Groynes In Geography
The Role Of Cell Surface Proteins In Dictating The Cellular Shape And The Morphological Appearance Of The Vascular Endothelium: The Appearance Of Fibronectin And Csp
A partial restoration of the normal morphology, adhesiveness, and contact inhibition of cell migration can be induced by adding fibronectin to a transformed culture. It has therefore, been suggested that fibronectin is involved in the control of cell morphology . However, in the case of vascular endothelial cells, fibronectin has no effect on their cellular organization, since its presence is neither correlated nor required for the formation of a monolayer composed of closely apposed and nonoverlapping cuboidal cells . This role can, however, be assigned to a major cell surface protein of 60,000 MW, when unreduced, and 30,000 MW when reduced. This cell surface protein is referred to as CSP-60. Under various normal and experimental conditions, CSP-60 showed a strict correlation with the establishment of a cell monolayer composed of highly packed, flattened, and nonoverlapping cuboidal or hexagonal cells. CSP-60 is present at confluence only in highly organized tissues, such as the vascular or corneal endothelium but not in cell types, such as vascular smooth muscle cells, that grow in three dimensions.
L. Vincent, A.J. Engler, in, 2017