Using Visuals To Help Explain Tonicity To Introductory Biology Students
BRIAN RAFFERTY is an Assistant Professor in the Science Department, Borough of Manhattan Community College, New York, NY 10007.
LALITHA JAYANT is a Professor in the Science Department, Borough of Manhattan Community College, New York, NY 10007.
Brian Rafferty, Lalitha Jayant Using Visuals to Help Explain Tonicity to Introductory Biology Students. The American Biology Teacher 1 March 2021 83 : 185187. doi:
The inability of students to properly understand the principles underlying osmosis and tonicity leads to misconceptions that further impair their ability to apply these concepts to physiological situations. We describe a simple and inexpensive visual exercise using beads and water to mimic solutions. Using these model solutions, students will understand the concepts of tonicity and osmolarity. The hands-on exercise is supplemented with a worksheet that reinforces the concepts they learned in doing the activity. This exercise has broad application with respect to both the level of students targeted and the courses in which it can be utilized, and it is flexible enough to personalize for each situation.
Why Osmosis Takes Place
Water diffuses through the semi-permeable membrane due to difference in diffusion pressure.
Diffusion pressure of pure solvent is higher while diffusion pressure of solution is lower due to addition of solute. Addition of solute lowers the diffusion pressure.
Water diffuses from a region of higher DP to lower DP.
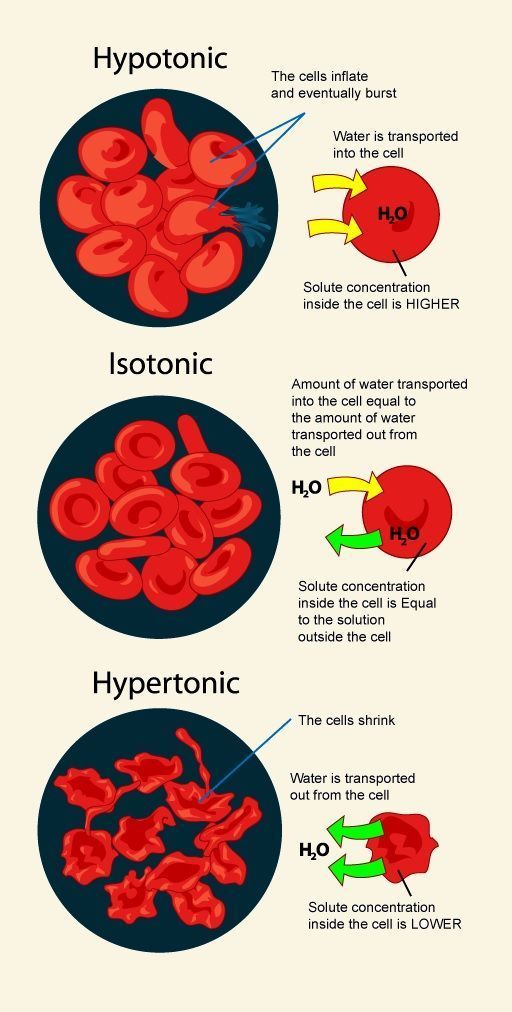
Effect Of Hypotonic Solution On Animal Cell
1. If an animal cell such as red blood cell is placed into a hypotonic solution, 2ater molecules is transported into the red blood cells by osmosis . 2. ¦he red blood cells will inflate and finally burst because the thin membrane cannot withstand the high pressure inside the cell. 3. ¦he red blood cells are said to undergo aemolysis. Effect of Hypotonic Solution on Plant Cell
1. chen a plant cell is placed in a hypotonic solution, water molecules is transported into te cell by osmosis. 2. ¦he water is then stored in vacuole causing it to expand and exerts pressure on the cell 2all. ¦his pressure is called turgor pressure. 3. ¦he turgor pressure caused the plant cell to become firm orturgid. 4. ¦he rigid cell 2all prevents cell from bursting.
If an animal cell such as red blood cell is placed into a isotonic solution, amount of 2atermolecules is transported into the red blood cells by osmosis is equal to the amount of watermolecules transported out from the cell . ¦herefore the amount of water in the cell remain unchanged. ¦he red blood cells maintain teir sape. Effect of Isotonic Solution on Plant Cell
1. chen a plant cell is placed in an isotonic solution, solute concentration in the external solution is equal to the solute concentration i the cell sap. 2. ¦herefore the rate of diffusion of water into the cell is equal to the rate of diffusion of water out from the cell. 3. As a result, the shape of the cell remain unchanged.
You May Like: What Math Do 9th Graders Take
What Happens To A Cell When Placed In A Hypotonic Solution
When we place a cell in a hypotonic solution, water gushes into the cell.
As already stated, the hypotonic solution contains less solute and more water than the cell interior. So, following the law of osmosis, water moves from its higher concentration to its lower concentration across the semi-permeable membrane. Consequently, the cytoplasm gains water, and the cell expands. As the cell membrane is freely permeable to water molecules, it allows the entry of water by endosmosis. That is why hypotonic solutions are crucial to the cells in case of cell dehydration, as it allows the entry of water into the cell.
If water influx continues past the permissible level, the osmotic balance of the cell might get disturbed. As a result, the cell will fail to hold the pressure, and its membrane will stretch to the limit that it lyses or bursts.
What Happens to RBCs in a Hypotonic Solution
RBCs, the oxygen carrier of blood, get bloated up and eventually burst when exposed to a hypotonic solution. Under any circumstances, it can hamper the oxygen transport in the body.
Is Diffusion Hypertonic Or Hypotonic
1: Red cell in hypertonic, isotonic, and also hypotonic services. Osmosis is the diffusion of water particles throughout a semipermeable membrane layer from a location of reduced focus service to a location of greater focus service .
What is hypotonic osmosis? The capacity of an extracellular service to make water relocate right into or out of a cell by osmosis is called itstonicity If the extracellular liquid has reduced osmolarity than the liquid inside the cell, its claimed to be hypotonic hypo suggests much less than to the cell, and also the internet circulation of water will certainly enjoy the cell.
Recommended Reading: Does Kamala Harris Have Any Biological Children
What Does Tonicity Mean In Biology
3.9/5Tonicity istonicity isthis is here
EXAMPLES. Tonicity is the reason why salt water fish cannot live in fresh water and vice versa. A solution with low osmolarity has a greater number of water molecules relative to the number of solute particles a solution with high osmolarity has fewer water molecules with respect to solute particles.
is water hypertonic or hypotonic? This more concentrated outside solution is termed hypertonic. In the last case, where the solution outside the cell has a lower solute concentration than the cell fluid, water will move into the cell towards the higher solute concentration. The less concentrated outside solution is termed hypotonic.
Keeping this in view, what is tonicity and how does it relate to Osmosis?
The ability of an extracellular solution to make water move into or out of a cell by osmosis is know as its tonicity. A solution’s tonicity is related to its osmolarity, which is the total concentration of all solutes in the solution.
How does exocytosis happen?
Exocytosis is the process of moving materials from within a cell to the exterior of the cell. In exocytosis, membrane-bound vesicles containing cellular molecules are transported to the cell membrane. The vesicles fuse with the cell membrane and expel their contents to the exterior of the cell.
What Is A Hypotonic Solution Example
A hypotonic solution is a solution that has a lower concentration of solute compared to the cell. The solute is the substance present in a lower amount, and the solvent is the substance present in greater amount. A hypotonic solution example is salt water. The salt is the solute, and the water is the solvent.
Also Check: Glencoe Geometry: Chapter 7 Test Answer Key
What Is A Hypotonic Solution Importent Definitions Chapter 5 Science Class 9 Notes Chapter 5 The Fundamental Unit Of
What is a hypotonic solution? chapter 5 can study by students of class 9. These definitiona and formulas of Class 9 Science Chapter 5: the Fundamental Unit of Life is developed and witten by our expert teachers. Science formulas. What is a hypotonic solution? is prepapred and collected from varius resources to help the students.
What is a hypotonic solution?
If the medium surrounding the cell has a higher water concentration than the cell, meaning that the outside solution is very dilute, the cell will gain water by osmosis. Such a solution is known as a hypotonic solution. Water molecules are free to pass across the cell membrane in both directions, but more water will come into the cell than will leave. The net result is that water enters the cell. The cell is likely to swell up.
What Is Tonicity In Biology
Tonicity is a procedure of the efficient osmotic stress slope the water possibility of 2 services divided by a semipermeable cell membrane layer. To put it simply, tonicity is the family member focus of solutes liquified in service which identify the instructions and also degree of diffusion.
What are the 3 sorts of osmosis? The 3 sorts of osmotic problems that impact living cells are called hypertonic, hypotonic, and also isotonic states. These terms define the osmotic state of the service that borders a cell, not the service inside the cell. Hypertonic problems create water to diffuse out of the cell, making the cell shrivel.
You May Like: What Does Commensalism Mean In Biology
What Is Hypotonic Solution
Hypotonicity is a relative word that describes a solutions properties in relation to another solution. In biology, the cytosolic fluid, or the fluid within a cell, is frequently used as a comparative solution. In biology, a solution is classified as hypotonic if it contains fewer solutes than a cells cytosol.
The semipermeable membrane is the cell membrane. So, what occurs when a cell is subjected to hypotonic conditions? A cell exposed to a hypotonic environment will experience an inflow of water, which will cause the cell to expand.
What Is Turgor Pressure
When a plant cell which is in a flaccid condition is immersed in water or a hypotonic solution then water moves into the cell by osmosis. As a result of the passage of water into the cell a pressure develops which prevails throughout the cell sap and is exerted against the protoplasm and the cell wall. This outward pressure that is produced due to entry of water is called as Turgor Pressure.
Read Also: Who Are Paris Jackson’s Biological Parents
Main Difference Isotonic Vs Hypotonic Vs Hypertonic
A solution is a homogeneous liquid mixture of two or more components. A solution is made by dissolving a solute in a solvent. There are three types of solutions grouped based on their concentrations. The concentration of a solution is the amount of solute present in a unit volume of the solution. The concentration of a solution determines its osmotic pressure the minimum pressure required to avoid a solution flowing through a semipermeable membrane. The main difference between isotonic hypotonic and hypertonic solutions is that isotonic solutions are solutions having equal osmotic pressures and hypotonic solutions are solutions having a lower osmotic pressure whereas hypertonic solutions are solutions with a high osmotic pressure.
The Salt In Sports Drinks
Ah, the classic debate: sports drinks versus water. While we will compare the two, we will look beyond which drink offers better hydration.
However, we must go to the debate to get to the crux of our matter. The big argument for the hydrating benefits of sports drinks, like Gatorade and PowerAde, is that they provide electrolytes, mainly in salts, to restore essential minerals lost in sweat. In so doing, they do more than hydrate because they balance more than the water in your body.
We will look at this factor in simpler terms. Because sports drinks are essentially a mixture of salt, vitamins, and minerals in water, they are a solution. The mixture is called the solute.
Plain water does not have the solute found in sports drinks, however. While science considers water a solution because it is a mixture of H2O molecules, it does not contain a mixture of extra salts, vitamins, and minerals. It is by lacking these extras that water is hypotonic to sports drinks.
Also Check: Electron Geometry Of Ccl4
Cells In Aqueous Solutions
The figures show what can happen when animal or plant cells are placed in an aqueous solution. Water can move across membranes, but polar solutes dissolved in water cannot. The net movement of water is in the direction of increased solute concentrations. An easy way to visualize this rule is simply that the net water movement is from an area of high water concentration to an area of low water concentration .
Animal cells
Why Is Ns Considered An Isotonic Solution
One liter of 0.9% saline has a of 154 mEq/L so the final osmolality is 308 mOsm. But this is the same osmolality as the water content of the blood. The measured osmolality of blood is lower because there is a 7% solid phase of blood that contains no NaCl. 0.9 saline is thus considered âisotonicâ.
Recommended Reading: What Is Figure Ground Perception Psychology
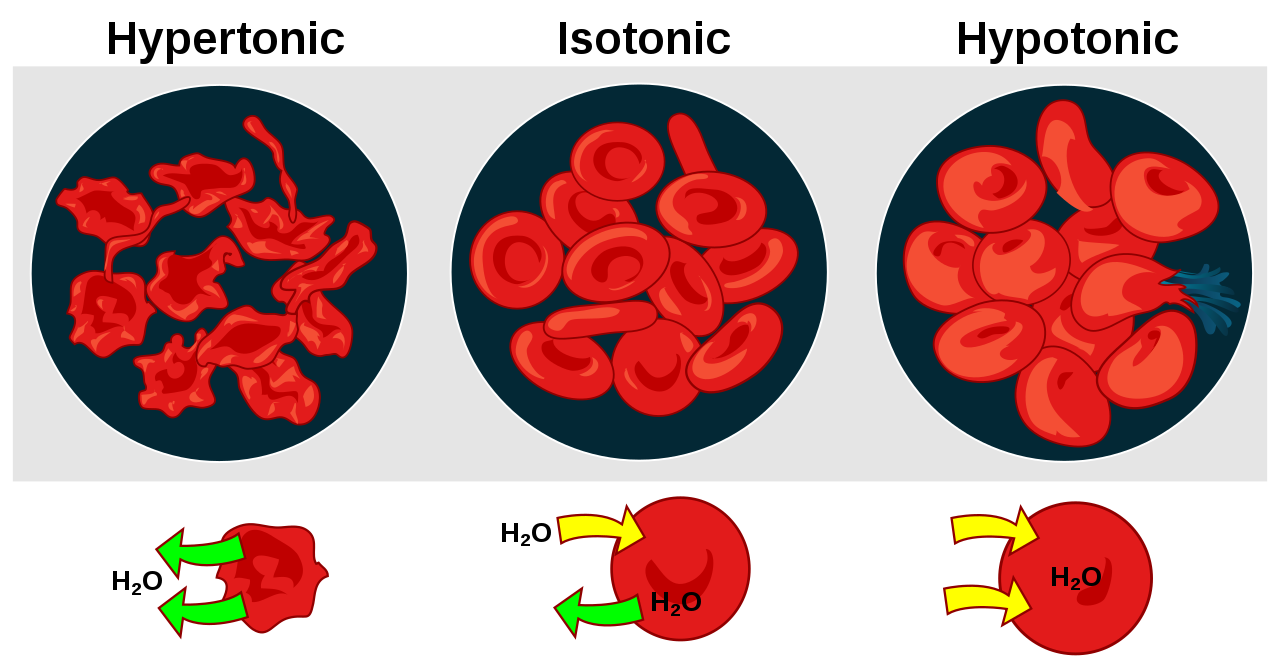
Key Differences Between Hypertonic And Hypotonic Solution
Both the hypertonic and hypotonic solutions are based on the concept of tonicity and osmosis. By knowing the concept of tonicity and osmosis, we can understand the general idea about the direction and extension of the water movement in the solution.
Difference Between Hypotonic And Hypertonic
July 9, 2012 Posted by Samanthi
The key difference between hypotonic and hypertonic is that hypotonic solution has a low solute concentration than the cell while hypertonic solution has a high solute concentration than the cell.
Osmosis is the process of moving water molecules from high water potential to low water potential through a semi-permeable membrane. However, this semi-permeable membrane only allows solvent particles to move across it and does not allow solute particles to move through the membrane. Tonicity is a measure of the osmotic pressure gradient and there are three states of it. These are hypertonic, isotonic and hypotonic. Among the three solutions, hypotonic solution is the solution which has a low solute concentration while hypertonic solution is the solution which has a high solute concentration. The solvent concentration gradient across the two solutions is the driving force for this process. The net movement of the solvent from hypotonic solvent to hypertonic solvent takes place due to the unequal osmotic pressure.
You May Like: Algebra Chapter 3 Test Answers
Why Is It Important In Cells
Cells always strive to maintain the equilibrium between their internal and external environments.
If the cell interior has a lower solute concentration , it confers that its extracellular medium has a higher concentration of solutes . Several essential cellular constituents, such as ions, electrolytes, and water, generally diffuse along their concentration gradients, i.e., from higher to lower concentration.
When the cell interior is hypotonic, water moves out of the cell, equalizing the solute concentration inside the cell and its surroundings.
Living cells can retain the rigid structure due to this regulation.
What Is The Difference Between Hypotonic And Hypertonic
A hypotonic solution is a solution that contains low solute concentrations while a hypertonic solution is a solution that contains high solute concentrations. So, this is the key difference between hypotonic and hypertonic. Besides, a hypotonic solution has a high water potential while a hypertonic solution has a low water potential. Therefore, this is also a significant difference between hypotonic and hypertonic solutions.
Moreover, a further difference between hypotonic and hypertonic solutions is that the water molecules move from a hypotonic solution to the cell while water molecules move from the cell to the hypertonic solution. Additionally, the cells shrink when placed in a hypertonic solution while the cells swell when placed in a hypotonic solution. Therefore, this is also an important difference between hypotonic and hypertonic.
The below info-graphic presents more information on the difference between hypotonic and hypertonic solutions, comparatively.
Also Check: Which Founding Contributors To Psychology Helped Develop Behaviorism
Yeast Respond To Hypotonic Shock With A Calcium Pulse*
- Laboratory of Genetics, University of Wisconsin-Madison, Madison, Wisconsin 53706Program in Cell and Molecular Biology, University of Wisconsin-Madison, Madison, Wisconsin 53706
- To whom correspondence should be addressed:AffiliationsLaboratory of Genetics, University of Wisconsin-Madison, Madison, Wisconsin 53706Program in Cell and Molecular Biology, University of Wisconsin-Madison, Madison, Wisconsin 53706
- Author Footnotes* This work was funded in part by a David and Lucile Packard Fellowship for Science and Engineering, by National Institutes of Health Grant GM48053 and NASA Grants NAGW-4053 and NAGW-4952 , and by a fellowship from the Arabidopsis Training Grant . This is Paper 3457 of the Laboratory of Genetics. The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Saccharomyces cerevisiaebisN,N,NN
What Is Osmotic Pressure
The accumulation of water in the left arm of the U-tube increases the hydrolytic pressure on the semipermeable membrane to stop further inflow of water from the right arm. This hydrostatic pressure is known as Osmotic Pressure .
The pressure required to prevent osmosis is termed as Osmotic Pressure .
Osmotic Pressure is also known as Osmotic Potential.
Unit of osmotic pressure is Atmosphere or Bars and symbol of osmotic pressure is PSI .
Read Also: Geometry Basics Segment Addition Postulate Worksheet Answer Key
Why Are Cells Hypertonic
Cells in Hypertonic Solutions In a hypertonic service the complete molar focus of all liquified solute fragments is above that of one more service, or above the focus in a cell. Because of this, water inside the cell will certainly stream in an outward direction to acquire balance, triggering the cell to diminish.
Elizabeth Martin And Robert Hine
- Publisher:
- or copy the link directly:https://www.oxfordreference.com/view/10.1093/acref/9780199204625.001.0001/acref-9780199204625-e-2204The link was not copied. Your current browser may not support copying via this button.Link copied successfully
date: 14 November 2021
Also Check: Ct Algebra 1 Curriculum Version 3.0 Activity 4.5 5 Answers
What Is A Hypotonic Solution
A solution is considered hypotonic if it contains a lower solute concentration or higher water content than another solution. The Greek word hypo stands for under or low, whereas tonic is derived from tonicity, which means relative concentration of a solution.
It is a relative term and can only be measured by comparing two solutions. Generally, it is compared to the cytosolic fluid .
Some widely used hypotonic solutions are freshwater, tap water, and normal saline .