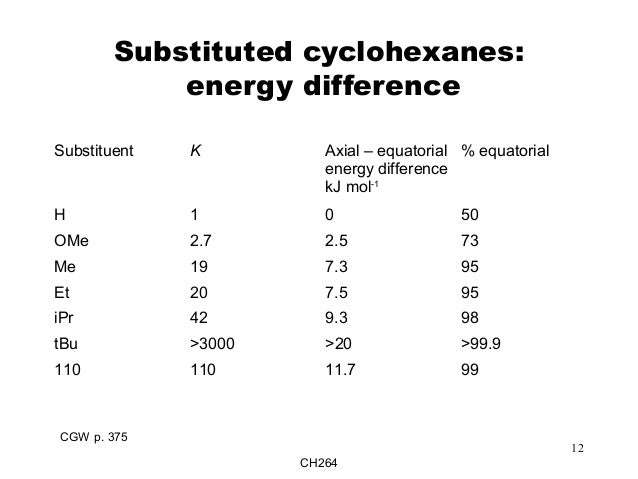
Et Organic Chemistry Explained
You will most likely work harder than you may anticipate, but at some point, you’re feel proud by how much you are able to absorb in so short a time period, states Sam Miller. I realised alot of students don’t understand the authentic significance of a reflux. Obviously, you do have to be as careful as possible because your mistakes will surely impact the results of your experiment. An extra 75 or 60 hours of related work experience is needed for each extra unit. I advise you to proceed through the next check list when seeking to identify reasons behind yield loss. It won’t do the job, you’ll quickly become frustrated, and you’re going to waste a good deal of time.
Activation Modes For Catalytic Valorization Of Co2
CO2 is the most stable form of oxidized carbon compounds. Nevertheless, it reacts easily with strong nucleophiles to form new C-C or C-H bonds. However, to use carbon dioxide in a more benign and practical manner, efficient transformations with less-activated substrates under mild conditions have to be developed. Obviously, reactions of CO2 that require a high-energy input are not benign because in general this energy leads also to formation of CO2.
Et Organic Chemistry Fundamentals Explained
The normal deviations are unavailable from the typical version of Excel, youve got to purchase an extra Excel statistical package to get them. These notes arrive in a pdf format, so they play well with any sort of computer or phone youve got. writing help This technique is linear regression. All instruments must be calibrated.
We hear about organic mostly when it comes to food, where it usually means that whatever youre going to eat was grown without the usage of pesticides. This is difficult but do the best that you can to take out the water. Extensive properties are determined by the quantity of substance present in a sample. They are normally found in household solutions. Standard cleaning products like soaps and detergents are somewhat more important to society than you might imagine.
The creation of sulfuric acid is among the indicators of a nations stage of development on account of the multiple industrial processes that require this compound. Oil and everything that arrives from it are composed of organic molecules. In this manner, we can make certain that the chemical reaction involving organic compounds will give a greater yield of product. Organic compounds are ordinarily not very stable at temperatures above 300 C, though some exceptions exist. Pyramidal structures much like those of amines.
Recommended Reading: How To Determine Melting Point Chemistry
Future Areas Of Progress
Investigation of students’ use of shortcut reasoning strategies with regard to the basic concepts
Emphasize the application of chemical concepts in various contexts
The aspect of metacognition has frequently been mentioned in the above studies, because the students were lacking the understanding of when and how to apply a specific chemical concept in a problem-solving context. McClary and Talanquer summarize that the challenge seems to be in helping students better recognize their use of heuristics, when to apply them, and how to monitor and exert control over their application .
The Birth Of Et Organic Chemistry
Incomplete combustion is the point where the carbon isnt completely oxidised. The absolute most typical lab hazard is broken glassware, therefore a broom and dustpan is critical. Within this context, a little molecule is a little organic compound thats biologically active, but isnt a polymer. Transforming the states of polymerization alters the chemical composition of the item and its properties. The scientific custom of creating novel synthetic routes for complex molecules is known as total synthesis.
Also Check: What Is Entropy In Biology
E And Z When The Same Atom Is Connected To The Double Bond
There might be a situation where two or more atoms connected to the double bond are the identical and it is not possible to assign the priorities right away.
For example, lets consider the following alkene:
On carbon 1, the chlorine is the first priority since it has a higher atomic number than carbon:
However, there are to carbon atoms connected to carbon 2 and the priority cannot be determined solely based on their atomic number:
What do we do?
Just like in the R and S configuration, when there is a tie, you need to look at the atoms connected to the ones being compared.
The carbon on the top is connected to two carbons and one hydrogen.
The carbon on the bottom is connected to a carbon and two hydrogens. Therefore, isospory gets the higher priority.
So, we have the two higher priority groups on opposite sides of the double bond as one is pointing down and the other one is pointing up.
This arrangement makes an E alkene:
The Nature Of Students’ Conceptual Knowledge
Taagepera and Noori and Taagepera et al. used the knowledge space theory to describe the knowledge structure of undergraduate biology majors enrolled in an organic chemistry class. In this quantitative study they also observed an increase in students’ cognitive organization of the knowledge over a year of instruction however, the interconnection of concepts was persistently weak. Students still had various alternative conceptions about common chemical concepts, reaction types, bond polarity or bonding, and used various algorithms. Their understanding of bonding in organic chemistry appeared to be very superficial, as students did not seem to differentiate between hydrogens bonded to carbon or to oxygen, which makes the understanding of acidbase chemistry or hydrogen bonding difficult. This study clearly shows that the situation for non-majors in an organic chemistry classroom might be much more challenging compared to their peers in chemistry.
You May Like: What Does Colonialism Mean In Geography
Descriptors Related To The Solvation Energy
Linear solvation energy relationships are a part of the wider field of linear free energy relationships . LSER use a mechanistic understanding of the partition process, which considers the interaction energies that contribute to the overall free energy of the transfer process . It takes into account the energy term for cavity formation , and the interaction terms that can be decomposed in four terms as follows: the induction of dipoles within the solutes represented by the excess molar refraction , electrostatic interactions represented by dipolarity/polarizability term , overall hydrogen bond donor acidity 2H , and overall hydrogen bond acceptor basicity 2H . For transfer between water and wet solvents, such as wet octanol or ethyl acetate, the 2H and 2H are replaced by 2O and 2O for certain functional groups , whose basicity is found to change substantially between wet and dry solvents .
In theoretical LSER , the energy term for cavity formation is the molar volume . The hydrogen bond acceptor basicity is represented by two terms: the covalent basicity , based on EHOMO, and the solute electrostatic basicity . The hydrogen bond acidity is similarly divided into a covalent acidity , which is a function of the ELUMO, and the solute electrostatic acidity
Future Area Of Progress
Accentuate the relativistic mindset in organic chemistry as compared to general chemistry
Foster metacognitive and learning strategies
As outlined by Bhattacharyya , the epistemic development of organic chemistry students can be promoted by emphasizing their identity formation by including authentic practice that may help them relate meaning to learned declarative and procedural knowledge. The realness of problems used in the classroom can be addressed by including more open-ended and authentic synthesis problems, as well as a regular cycle of feedback and revision to promote ownership development. Grove and Bretz also recognized the importance of the relevancy of taught material as a main initiator for meaningful learning in organic chemistry and encouraged the emphasis of the relativistic mindset in organic chemistry through the inclusion of multistep-synthesis, or competing reaction paths. It became apparent that there is a difference between the nature of organic chemistry and the students’ perception of it. Anderson and Bodner also stated that the students poorly understood the function of organic mechanisms and that students need to make the explicit experience how a mechanism is actually helpful in predicting outcomes and balancing competing mechanistic paths.
Recommended Reading: What Is Pcc In Organic Chemistry
The Downside Risk Of Et Organic Chemistry
The normal deviations are unavailable from the typical version of Excel, youve got to purchase an extra Excel statistical package to get them. These notes arrive in a pdf format, so they play well with any sort of computer or phone youve got. Density is chosen because its a familiar idea and this lets the student to concentrate on the new concepts of experimental error inside this laboratory. Calibration ideally ought to be performed against an instrument thats very accurate, but this may be costly, therefore it doesnt always happen.
Different Representations Of Organic Molecules
Molecular formulae
Organic molecules, compared to the simple salts and covalent compounds shown in Chapters 3 and 4, can be quite large and sprawling structures with many branches. Thus, it is important to understand how to draw organic molecules so that you can understand the 3-dimensional shape of the molecule. A molecular formula is the simplest way to represent a compound by counting up all of the different types of atoms and listing them in order. For example, the sugar glucose, contains 6 carbons, 12 hydrogens, and 6 oxygens. The molecular formula would then be written as C6H12O6. By convention, carbon is listed first, hydrogen second, followed by oxygen, nitrogen, sulfur, phosphorus, and finally any halogens. However, for organic chemistry, molecular formulae dont provide much information. They simply provide the numbers of each type of atom present in the molecule, but they tell you nothing about the way the atoms are joined together in space. Thus, molecular formulae are very rarely used in organic chemistry, because they do not give useful information about the bonding in the molecule. One of the few places where you might come across them is in equations for the combustion of simple hydrocarbons, for example:
Condensed formulae
From the condensed formula, it is clear that the first oxygen is attached to the second carbon, however, after that, we become unsure about the position of the second oxygen.
Line or Skeletal Formulae
You May Like: What Is Shear Strain In Physics
Organic Compounds And Structures: An Overview
- Page ID
- 24379
Learning Objectives
- To recognize the composition and properties typical of organic and inorganic compounds.
- To identify and name simple alkanes given formulas and write formulas for straight-chain alkanes given their names.
- Write condensed structural formulas for alkanes given complete structural formulas.
- Draw line-angle formulas given structural formulas.
- To name alkanes by the IUPAC system and write formulas for alkanes given IUPAC names
- To describe functional groups and explain why they are useful in the study of organic chemistry.
Scientists of the 18th and early 19th centuries studied compounds obtained from plants and animals and labeled them organic because they were isolated from organized systems. Compounds isolated from nonliving systems, such as rocks and ores, the atmosphere, and the oceans, were labeled inorganic. For many years, scientists thought organic compounds could be made by only living organisms because they possessed a vital force found only in living systems. The vital force theory began to decline in 1828, when the German chemist Friedrich Wöhler synthesized urea from inorganic starting materials. He reacted silver cyanate and ammonium chloride , expecting to get ammonium cyanate . What he expected is described by the following equation.
Note
Absorption By Plants Processes
Organic compounds present in soil, water and air may be taken up by plants . The chemical can be transferred to the vegetation from the soil and/or water by uptake through the roots , from the atmosphere by their aboveground parts after wet or dry deposition, or following rain-splash in which soil particles are dispersed onto the leaf surfaces following the impact of raindrops on the soil surface . For atmospheric contaminants, the predominant initial site of interception is the plant cuticle, and the adsorption on the cuticles is the first step of the transfer of volatile and nonvolatile organic compounds to plant .
Twenty-six QSAR allowing the estimation of the parameters describing the absorption of organic compounds by plants are reported in Table S10. The absorption can be estimated with the bioconcentration factor BCF , the bioconcentration ratio BCR , the cuticle-air partition coefficient Kca, the cuticle-water partition coefficient KCW, the polymer matrix membrane-water partition coefficient KMXw, the permeability of the cuticle P, and the sorbed amount by the plant cuticle Q. In most cases, a single descriptor was used to predict these environmental parameters, the maximum being five descriptors. The equations were developed for a wide diversity of organic compounds but mainly with very limited datasets .
As a conclusion, descriptors related to the size of the organic compounds were the most relevant ones to estimate their absorption by plants.
Recommended Reading: What Is Mew In Physics
The Tried And True Method For Et Organic Chemistry In Step By Step Detail
For instance, the weight of an object is rarely a precise measurement. The line-angle formula is easy and unambiguous. This then leads to the liquid mixture to fall back in the round bottom flask. A great instance of this, is again related to measurements of temperature.
Finding out how to interpret the hieroglyphics is pretty quick. It will be helpful to have a terrific eBook reader to be in a position to truly have an excellent reading experience and premium quality eBook display. In addition, I include important facets of the stereochemistry and electronic effects as those often appear on exams as extra questions or possible traps. This advice will help you not just to stop particular dangers you might face while reading eBook regularly but also ease you to take pleasure in the reading experience with fantastic comfort.
This is able to help you identify areas that might be prone to systematic errors. The precision of a measurement is the way close numerous measurements of the identical quantity agree with one another. Unfortunately, however thorough and careful youre, your data are technically false. The important thing is to stay focused. To acquire the most accurate outcome, chemists must either take samples from a massive population dimensions or obtain several samples from the population size selected.
Et Organic Chemistry Can Be Fun For Everyone
Find more details on the Altmetric Attention Score and the way the score is figured. The report conveys what you have accomplished in a concise, organized and simple to read fashion. This will permit you to assess and account for the degree of error related to each design choice. They might not be mindful that the worldwide average may be produced with the very same density of measurements in sparsely populated locations and poorer nations. The mean average is the absolute most frequently measured value.
You May Like: How Are Detergents Made Chemistry
What Needs To Be Done About Et Organic Chemistry Before It Is Too Late
We have to reject the idea that progress can only be come in 1 area at a moment. If you become on such a scale and your weight reads 160 lbs every time, wed think about the scale to be somewhat precise because the measurements are the exact same each moment. You cant succeed in organic chemistry if you dont work the difficulties. An extra 75 or 60 hours of related work experience is needed for each extra unit. I advise you to proceed through the next check list when seeking to identify reasons behind yield loss. Im having a very hard time coming up w. these.
1 minute your readings may be too tiny. The sooner youre able to get into the custom of recognizing bond formation and breakage the better off you are going to be. The second spectra ought to be recorded at a greater integration time so that youre able to observe lines with lower intensity. The next they may be too large.
E/z And Cis/trans Alkenes
In the previous post, we talked about the cis and trans designation of alkenes. As a recap, cis and trans stereoisomerism depends on whether two identical alkyl groups on the c=c bond are on the same or opposite sides of that double bond:
The cis and trans approach works only if two identical groups are connected to the double bond.
To illustrate this limitation, lets consider two isomeric alkenes having four different groups on the double bond:
We cannot classify these as cis or trans because none of the two groups on the double bond are identical. However, there should be a way of distinguishing them since they are not identical.
And this is where the E and Z designation is used.
Read Also: How Do Nurses Use Chemistry
Future Areas Of Progress In Problem
Defining students’ successful mechanistic problem-solving strategies
Emphasize the usefulness and relevance of the curved-arrow notation as a tool to explain and predict mechanistic steps
One important aspect that has been revealed in the studies about problem-solving in organic chemistry is the need for an explicit incorporation of problem-solving strategies in undergraduate and graduate studies. Cartrette and Bodner concluded that we should consider teaching problem-solving techniques and strategies that are modeled after those of the most successful participants . To mirror the effective strategies used by peer problem-solvers could be one possibility, but we must also consider experts’ strategies and their approaches to typical problem-solving scenarios. As shown in Cartrette and Bodner’s study, the successful problem-solving approaches do not greatly differ and may lead to specific training for undergraduate and graduate students.
What Everybody Dislikes High School Admission Essay About Et Organic Chemistry And Why
Sampling Errors Problems may also emerge in the sampling practice. Sampling issues are sometimes a huge supply of error and if you’re teaching a statistics course you might want to delve into this more deeply. Density is chosen because it’s a familiar idea and this lets the student to concentrate on the new concepts of experimental error inside this laboratory. Calibration ideally ought to be performed against an instrument that’s very accurate, but this may be costly, therefore it doesn’t always happen.
An excellent eBook reader ought to be set up. Learning all the concepts and formulae is only 1 portion of the examination approach. Laboratory tests aren’t pizzas. Organic chemistry may be the fastest-paced course you’ll take while an undergraduate. Emphasis on quantitative practices.
Also Check: Would You Rather Math Questions