Oxidation Of Primary Alcohols
The mechanism of oxidation involves an alpha hydrogen . As a primary alcohol has two alpha hydrogen atoms two steps of oxidation are possible. The first step of oxidation produces an aldehyde whereas the second step of oxidation produces a carboxylic acid. Note that each step of oxidation leads to the loss of an alpha hydrogen and an increase in the number of bonds to oxygen.
What Does Pcc Do In Organic Chemistry
PCCdoesPCC
. Simply so, what is PCC used for in organic chemistry?
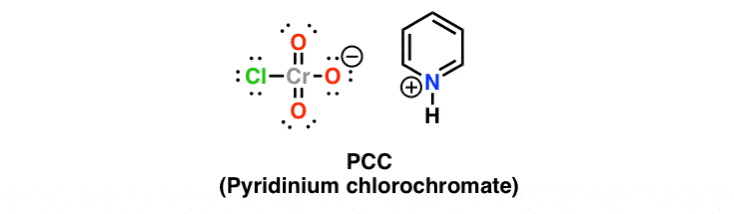
Pyridinium chlorochromate is a yellow-orange salt with the formula +. It is a reagent in organic synthesis used primarily for oxidation of alcohols to form carbonyls. A variety of related compounds are known with similar reactivity.
Similarly, what is the structure of PCC? C5H5NHClCrO3
In respect to this, is PCC a reducing agent?
Pyridinium chlorochromate is a weak oxidizing agent and is often used to oxidize alcohols into carbony compounds. All of the other compounds are similar in that they function as reducing agents.
What does the Jones reagent test for?
Jones reagent is a solution of chromium trioxide in aqueous sulfuric acid. Using acetone as a reaction solvent, the reagent is usually used for the oxidation of primary and secondary alcohols to carboxylic acids and ketones, respectively.
Which Is More Selective Pcc Or Collins Reagent
PCC offers the advantage of the selective oxidation of alcohols to aldehydes or ketones, whereas many other reagents are less selective. This complex is both difficult and dangerous to prepare, as it is very hygroscopic and can inflame during preparation. It is typically used in a sixfold excess in order to complete the reaction.
Also Check: Elton John Kids Adopted
How Does Pcc Not Oxidize Aldehyde
I am wondering how does PCC actually work in not oxidizing aldehyde. Like the reaction mechanism I saw online, it seemed as if not having a acidic solvent is what made aldehyde not tranform to carboxylic acid when PCC is present. So the why can’t you just use chromium containing compounds without the acidic solvent and then you will be able to isolate aldehyde.
- $\begingroup$The crux lies in the half-equation for the reduction of PCC. It must have a source of $\ce$ ions for any reaction to take place in the first place.$\endgroup$
The “trick” is in the enviroment, not in the PCC itself.
Alcohols tend to be oxidized to carboxylic acids when they are in aqueous enviroment.
PCC was invented as a workaround to this problem: it works as a reactant in anhydrous enviroment, hence stopping the reaction at the aldheyde stage.
An alternative way to see PCC is to follow its preparation: if you add $\ce$ and pyridine, PCC is formed.If you add $\ce$, acid and water, chromic acid is formed.
So, you see that the reagents are similar: the point is in finding at which stage the difference is.
Mechanistically speaking, the difference occurs as follows: in aqueous enviroment, water can form a geminal diol intermediate by attacking the aldheyde. This step adds the second oxygen to the aldheyde that, with further reaction with chromic acid, yelds a carboxylic acid.
When water is not present, this step doesn’t occur, and the reaction stops.
Recent Advances In Application Of Pyridinium Chlorochromate In Organic Synthesis
Volume 13 , Issue 2 , 2016
Page: Pages: 35
Abstract
Pyridinium chlorochromate is an important reagent in organic synthesis used primarily for the selectiveoxidation of alcohols to give carbonyl compounds. Although a variety of related compounds are knownwith similar reactivity, PCC offers exclusively the advantage of the selective oxidation of alcohols to aldehydes,whereas many other reagents were less selective. Disadvantages of using PCC are the tedious reaction workup andits toxicity, very well compensated by selective oxidation, observed using this reagent as an oxidant. This usefuloxidant was first synthesized and used by E. J. Corey and J. William Suggs in 1972.
Title:Recent Advances in Application of Pyridinium Chlorochromate in Organic Synthesis
Volume: 13Issue: 2
Majid M. Heravi, Azadeh Fazeli and Zeinab Faghihi
Affiliation:
Keywords:Pyridinium chlorochromate , organic synthesis, alcohol, oxidation, carbonyl compounds, selectiveoxidation.
Read Also: What Does The Prefix Mito Mean
Course Details: Crn 10558
A printer is required for this course.
Instructor comments:
This information was provided to all CH 241 students in Fall 2021. For those who took Organic Chemistry I at another institution, this information will be particularly important.
There are five main items you need to purchase for the class:
I would recommend a physical text to all students taking the complete 241-2-3 series and/or those who will need to review Organic Chemistry at a later date . Once the 24 month period expires for the ebook, you will no longer have access.
Definition: What Is Pcc Reagent
PCC reagent is used in the organic synthesis of aldehyde and ketone from primary and secondary alcohols, respectively. It is a yellow-orange colored salt with the formula +. PCC oxidizes the alcohol group and does not affect any other functional group or double bond present in the compound .
The history of this reagent goes back to 1975 when E J Corey and W Suggs suggested it as an oxidizing agent.
Read Also: What Is The Molecular Geometry Of Ccl4
Why Is Pcc Toxic
However, PCC was probably avoided due to its toxicity from Chromium . Similar to the problem with PCC, it is also toxic due to the presence of Chromium , if using chromium trioxide. A reaction with Jones reagents also occurs in acidic conditions, which could cause damage as well if handled improperly.
Pyridinium Chlorochromate And Dichromate
An alternative to the chromium trioxidepyridine complex is provided by pyridinium chlorochromate and pyridinium dichromate .137 These reagents, now ubiquitous for chromate-based oxidation of alcohols, overcome the hygroscopic nature of the chromium trioxidepyridine complex138 and are prepared by a less hazardous procedure 139 both are commercially available as are several other derivative reagents.
Pyridinium chlorochromate has been shown to be of particular value in the allylic oxidation of compounds containing an activated methylene group, such as 5,6-dihydropyrans .140
Indeed, Parish141 claims that PCC is the reagent of choice in the allylic oxidation of 5-steroids . The reactions were carried out using PDC in pyridine solution at 100 °C, PCC in refluxing benzene solution, and PCC in DMSO solution at 100 °C. These solvent systems are claimed to be superior to the more usual methylene chloride.138,142
One drawback associated with this type of chromium species is the frequent requirement for a large excess of reagent. Recent attempts to combat this problem have involved the use of a PCCcelite mixture in benzene solution under reflux143 and more successfully a t-butyl hydroperoxidepyridinium dichromate mixture .144
Kristine L. Teppang, … Jerry Yang, in, 2020
Read Also: Does The Mcat Give You Equations
Synthesis Of Analogs Starting From Diosgenin
Shawakfeh and coworkers described the preparation of several structurally simplified analogs of cephalostatin starting from diosgenin . In the first approach, CaCO3-buffered oxidation of 247 with PCC afforded the unsaturated ketone 248, which was brominated with PTAB in THF to produce the unsaturated 2-bromoketone 249. Treatment of 249 with NaN3, and KI in dimethylformamide , followed by reduction of the azide group and TsOH-catalyzed condensation afforded the symmetrical analog 250. Application of the protocol for the construction of the pyrazine core to the unsaturated diketone 251, obtained by Jones oxidation of diosgenin , afforded the analog 253 .90
Scheme 2.39. Synthesis of tridecacyclic pyrazines starting from diosgenin.
Hydrogenation of the unsaturated ketones 248 and 251 over Pd and application of the same protocol for the generation of the pyrazine core afforded the corresponding analogs 254 and 255 .
Figure 2.8. Tridecacyclic pyrazines bearing saturated A, A-rings.
Scheme 2.40. Synthesis of a 5,5,6,6-tetrahydroxy-tridecacyclic pyrazine starting from diosgenin.
Epoxidation of the unsaturated diketone 251 followed by the same synthetic sequence as shown in Schemes 2.39 and 2.40 afforded the epoxidized analog 261 .91
Scheme 2.41. Synthesis of a 5,6,5,6-diepoxy-tridecacyclic pyrazine starting from diosgenin.
Scheme 2.42. Synthesis of a 5,5-dihydroxy-6,6-dioxo-tridecacyclic pyrazine starting from diosgenin.
Amitava Dasgupta, in, 2017
What Is Pcc In Organic Chemistry
Answer:
Pyridinium chlorochromate is a yellow-orange salt with the formula +. It is a reagent in organic synthesis used primarily for oxidation of alcohols to form carbonyls. A variety of related compounds are known with similar reactivity.
Pyridinium chlorochromate is a yellow-orange salt with the formula +. It is a reagent in organic synthesis used primarily for oxidation of alcohols to form carbonyls. A variety of related compounds are known with similar reactivity.Appearance: yellow-orange solid
Pyridinium chlorochromate is a yellow-orange salt with the formula +. It is a reagent in organic synthesis used primarily for oxidation of alcohols to form carbonyls. A variety of related compounds are known with similar reactivity.Appearance: yellow-orange solidChemical formula: C5H6ClCrNO3
Pyridinium chlorochromate is a yellow-orange salt with the formula +. It is a reagent in organic synthesis used primarily for oxidation of alcohols to form carbonyls. A variety of related compounds are known with similar reactivity.Appearance: yellow-orange solidChemical formula: C5H6ClCrNO3Solubility in other solvents: soluble in acetone, acetonitrile, THF
Pyridinium chlorochromate is a yellow-orange salt with the formula +. It is a reagent in organic synthesis used primarily for oxidation of alcohols to form carbonyls. A variety of related compounds are known with similar reactivity.
Appearance: yellow-orange solid
You May Like: Fsa Algebra 1 Eoc Algebra And Modeling Answer Key
Ch242 Organic Chemistry Ii
Introduces radical reactions substitution and elimination reaction mechanisms structure and chemistry of alcohols, ethers, epoxides and their sulfur analogues organometallic compounds arenes and aromaticity structure and chemistry of aromatic compounds NMR, UV-VIS and Mass Spectroscopy and special topics as time and interest permit. Prerequisite: CH 241. Audit available.
- you will need the CRN to register.
- Fees:
- many classes have additional fees.
- Materials:
- classes marked with $0 or < $40 use low cost materials. Cost does not include art supplies, calculators, fees, and equipment.
- in-person: remote: online: hybrid:
| CRN |
|---|
| Notes: CRN includes Lec & Lab. |
| and SY Remote |
This page includes one section only, more sections may exist for this class.
Mechanism For The Oxidation Of Primary Alcohols To Aldehydes With Pyridinium Chlorochromate
How does it work? Oxidation reactions of this sort are actually a kind of elimination reaction. Were going from a carbon-oxygen single bond to a carbon-oxygen double bond. The elimination reaction can occur because were putting a good leaving group on the oxygen, namely the chromium, which will be displaced when the neighboring C-H bond is broken with a base.
The first step is attack of oxygen on the chromium to form the Cr-O bond. Secondly, a proton on the OH is transferred to one of the oxygens of the chromium, possibly through the intermediacy of the pyridinium salt. A chloride ion is then displaced, in a reaction reminiscent of a 1,2 elimination reaction, to form what is known as a chromate ester.
The C-O double bond is formed when a base removes the proton on the carbon adjacent to the oxygen. The electrons from the C-H bond move to form the C-O bond, and in the process break the O-Cr bond, and Cr becomes Cr in the process 2 ).
Real life notes: If you end up using PCC in the lab, dont forget to add molecular sieves or Celite or some other solid to the bottom of the flask, because otherwise you get a nasty brown tar that is a real major pain to clean up. The toxicity and mess associated with chromium has spurred the development of other alternatives like TPAP, IBX, DMP, and a host of other neat reagents you generally dont learn about until grad school.
References and Further Reading
Also Check: Qualitative Data Definition Ap Human Geography
Why Is Pyridinium Chlorochromate Selective Compared To Chromate Dichromate Etc
One can use PCC to oxidise an alcohol selectively up one level – to an aldehyde/ketone, without further oxidation to a carboxylic acid.Why is pyridinium important to this selectivity?
I understand that usually PCC is dissolved in dichloromethane and not in anything like THF, and this prevents the formation of a hydrate, which can then act “like” an alcohol in it’s oxidation to a carboxylic acid, but I can’t figure out why it’s important that pyridinium is used.
Though pyridinium chlrochromate is a salt, the pyridnium cation is easily dissolved by a wide variety of organic solvents. As such, the chlorochromate anion then becomes dissolved in the organic solvent, and can oxidize $1º$ and $2º$ alcohols present in solution to carbonyls. Oxidation of carbonyls to carboxylic does not occur when using PCC for the very reason you said yourselfno water is present to hydrate the carbonyl species to its geminal diol to allow for further reaction.
Other benefits of PCC include that it is not particularly hygroscopic, is commercially available, and can be stored for some time, though other methods are generally preferred as the reaction workup for PCC can be tedious and the chromium species produced is toxic.
Jones Reagent Versus Pcc
In organic chemistry students will learn both Jones and PCC reagents and will need to know how to differentiate between the two. Both reagents oxidize alcohols. Only secondary and primary alcohols can be oxidized, since oxidizing tertiary alcohol will lead to a carbon with five bonds which is impossible. Both Jones reagent and PCC turn a secondary alcohol into a ketone, going from C-OH to C=O. The difference comes to the primary alcohols. PCC turns a primary alcohol into an aldehyde . However, Jones reagent is strong and oxidizes primary alcohol further to carboxylic acids .
What other reagents produce C=O? Ozonolysis . Ozonolysis is done on alkenes. It can be under reductive and oxidative conditions. Under reductive conditions, O3/DMS, all we need to do is break the double bond and attach an O on each side. Therefore, reductive ozonolysis will create ketones and aldehydes. Ozonolysis under oxidative contusions, on the other hand, will lead to carboxylic acids instead of aldehydes.
DMP is the final reagent we will discuss here. DMP is used to oxidize primary alcohols to aldehydes and secondary alcohols to ketones. Therefore DMP is similar to PCC in the end results it gives.
Let Transformation Tutoring help you ace your organic chemistry class and find passion and love for the subject. Our organic chemistry tutors in Brooklyn, NYC, and online are here to help you and talk to you. Please call 646-407-9078 and we will gladly discuss your needs.
You May Like: What Is An Elastic Force
Can Toluene Be Oxidized To Benzoic Acid Using Pyridinium Chlorochromate
PCC is generally regarded as a milder oxidizing agent, used to effect selective oxidation of alcohols to aldehydes or ketones . While over-oxidation to a carboxylic acid from a primary alcohol is definitely possible, PCC should not react with a simple unfunctionalized benzylic carbon.
However, oxidation at the benzylic position is easily achieved with more aggressive oxidizing agents. In particular, chromic acid as well as aqueous potassium permanganate will fully oxidize virtually any benzylic carbon all the way to the carboxylic acid, providing the benzylic carbon has at least one hydrogen. Of course, the rest of the chain is completely cleaved in the process.
Edit: Out of curiosity, I consulted my copy of March’s Advanced Organic Chemistry to determine if any other common reagents were able to effect the same benzylic oxidation. Nitric acid is listed along with the two methods I described above. Naturally, a survey of various more exotic methods is also given. Intriguing was the use of sodium hypochlorite in acetonitrile, or NBS in aqueous sodium hydroxide under photochemical conditions, for the specific complete oxidation of aryl methyl groups.
Are Hydrogen And Hydronium The Same
What is the difference between Hydrogen Ion and Hydronium Ion? Hydrogen ion is shown by the symbol H+ and hydronium ion is denoted by the symbol H3O+. Hydrogen ion is obtained by removing an electron from the hydrogen atom. Since this is so reactive, in aqueous medium it combines with water, to form a hydronium ion.
Read Also: Domain And Range Worksheet 2 Answer Key Algebra 1
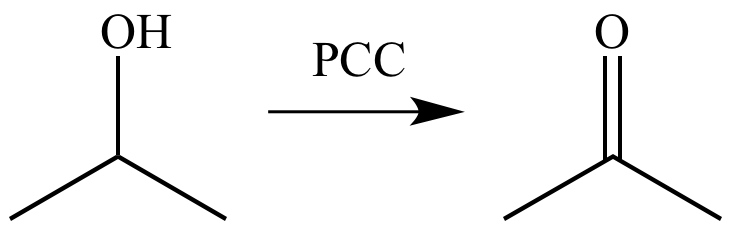
Oxidation Of Primary Alcohols To Aldehydes With Pyridinium Chlorochromate And Oxidation Of Secondary Alcohols To Ketones
Here are two examples of PCC in action. If you add one equivalent of PCC to either of these alcohols, you obtain the oxidized version. The byproducts are Cr as well as pyridinium hydrochloride.
One has to be careful with the amount of water present in the reaction. If water is present, it can add to the aldehyde to make the hydrate, which could be further oxidized by a second equivalent of PCC were it present. This is not a concern with ketones, since there is no H directly bonded to C.
Why Is Pcc Needed
Police Clearance Certificate is issued to Indian Passport holders in case they have appped for Residential Status, Employment or Long term visa or for immigration. PCC cannot be issued for persons going abroad on Tourist Visa. Q: What are the documents required to apply for a PCC in India ?Dec 28, 2019.
Recommended Reading: Segment Addition Postulate Unit 1 Geometry Basics