First Law Of Thermodynamics In Biological Systems
All biological organisms require energy to survive. In a closed system, such as the universe, this energy is not consumed but transformed from one form to another. Cells, for example, perform a number of important processes. These processes require energy. In , the energy is supplied by the sun. Light energy is absorbed by cells in plant leaves and converted to chemical energy. The chemical energy is stored in the form of glucose, which is used to form complex carbohydrates necessary to build plant mass.
The energy stored in glucose can also be released through cellular respiration. This process allows plant and animal organisms to access the energy stored in carbohydrates, lipids, and other macromolecules through the production of ATP. This energy is needed to perform cell functions such as DNA replication, mitosis, meiosis, cell movement, endocytosis, exocytosis, and apoptosis.
Entropy Balance Equation For Open Systems
In chemical engineering, the principles of thermodynamics are commonly applied to “open systems“, i.e. those in which heat, work, and mass flow across the system boundary. Flows of both heat ( Q T is the absolute thermodynamic temperature of the system at the point of the heat flow. If there are mass flows across the system boundaries, they also influence the total entropy of the system. This account, in terms of heat and work, is valid only for cases in which the work and heat transfers are by paths physically distinct from the paths of entry and exit of matter from the system.
To derive a generalized entropy balanced equation, we start with the general balance equation for the change in any extensive quantity θ in a thermodynamic system, a quantity that may be either conserved, such as energy, or non-conserved, such as entropy. The basic generic balance expression states that d , i.e. the rate of change of θ in the system, equals the rate at which θ enters the system at the boundaries, minus the rate at which θ leaves the system across the system boundaries, plus the rate at which θ is generated within the system. For an open thermodynamic system in which heat and work are transferred by paths separate from the paths for transfer of matter, using this generic balance equation, with respect to the rate of change with time t of the extensive quantity entropy S , the entropy balance equation is:
- d
- \ln }},}
- Î
- }=}}}}}.}
When Water Freezes What Happens To Entropy
At all temperatures, the entropy of water molecules decreases upon freezing because water molecules are more ordered in the crystalline state than in the liquid. Water molecules have more rotational and translational freedom in liquid than in the solid. So Ssys always pushes ice to water.
Read also
You May Like: What Is Elastic Force
Energy Storage In Cells
Although all organisms use ATP as the immediate free-energy source in biochemical reactions, ATP is not an efficient form in which to store energy on a long-term basis. If the caloric intake of an average resting adult human were stored as ATP, two-thirds of the body weight would have to consist of ATP. Instead, a typical 70 kg adult human has a total of only about 50 g of both ATP and ADP, and, far from being used for long-term storage, each molecule of ATP is turned over about 860 times per day. The entire ATP supply would be exhausted in less than 2 minutes if it were not continuously regenerated.
Figure 18.21 Percentages of Forms of Energy Storage in Adult Humans
An average 70 kg adult stores about 6 × 105 kJ of energy in glucose, glycogen, protein, and fats. Fats are the most abundant and most efficient form for storing energy.
Books About Entropy And Biology 1980
Abusing Science
Another typical example of confusion between the two kinds of entropy comes from a similar book by Tim M. Berra, Evolution and the Myth of Creationism. The following paragraph from that book would seem to indicate that any large animal can assemble a bicycle .
For example, an unassembled bicycle that arrives at your house in a shipping carton is in a state of disorder. You supply the energy of your muscles to assemble the bike. You have got order from disorder by supplying energy. The Sun is the source of energy input to the earth’s living systems and allows them to evolve.
A rare example of the use of mathematics to combine the two kinds of entropy is given in The Mystery of Life’s Origin, published in 1984. Its authors acknowledge two kinds of entropy, which they call “thermal” and “configurational.” To count the “number of ways” for the latter kind of entropy they use restrictions which they later admit to be unrealistic. They count only the number of ways a string of amino acids of fixed length can be sequenced. They admit in the end, however, that the string might never form. To impose the units joules per degree onto “configurational” entropy, they simply multiply by Boltzmann’s constant . Nevertheless, they ultimately reach the following conclusion :
Roger Penrose’s treatment of entropy is worth mentioning. In The Emperor’s New Mind, he nimbly dodges the problem of assigning physical units to logical entropy :
| Penrose |
| Prigogine |
| Adami |
alwaysplus
You May Like: Operations With Complex Numbers Kuta Software
What Is Entropy An Historical Perspective
Rudolf Clausius coined the term entropy from the Greek word Entropein for transformation and change . This transformative idea arose from studying the interaction of refrigeration and a heat engine and the transfer of the heat, Q, between the two. Clausius’s conclusion was that heat changed while the quantity of the ratio of the heat over the temperature, T, remained the same. This conclusion led to the first definition that described the change of entropy as:
In words, Equation states that the change in entropy with time will always be greater or equal to the change of heat divided by the temperature, or put more simply using Equation , the change of entropy with time will always be greater or equal to the starting entropy. But the question still remained, what was this mysterious quantity known as entropy? Clausius’s definition did not state what entropy was but only how entropy changed as a function of heat and temperature. It was clear that entropy had an innate tie with the degradation of usable energy into an unusable form. From an engineering perspective, this meant the generation of unrecoverable heat from any work done by an engine or person .
When Is Entropy Increases What Happens To Enthalpy
Besides, when entropy increases what happens to enthalpy? offcourse the molecules will collide at higher rate with one another and hence the disorder is increases , so the entropy is increased. In contrast, enthalpy is the heat that is contained in the system or body, in above example the heat that forces the molecules to collide one another is called Enthalpy .
Don’t Miss: Beth Thomas Interview
What Is The Entropy Cost When Two Molecules Form A Complex
Biology is driven by molecular interactions. Our understanding of the constant flux back and forth between molecules with different identities is largely a story about free energy differences between reactants and products as all science students learn in their first chemistry course. However, the cursory introduction to these matters experienced by most students casts aside a world of beautiful subtleties that center on the many ways in which the free energy of a molecular system is changed as a result of molecular partnerships. Here we focus on the contribution to the free energy resulting from the entropy changes when molecules bind.
In this vignette, we address a simple conceptual question, namely, when two molecules A and B interact to form the complex AB, how large is the entropy change as a result of this interaction? The free energy has the generic form
G=H-TS,
where H is the enthalpy and S is the entropy.
We see that in a simple case in which there is no enthalpy change, the entire free energy balance is dictated by entropy. If a reaction increases the entropy this means there is a corresponding negative free energy change, signaling the direction in which reactions will spontaneously proceed. A deep though elusive insight into these abstract terms comes from one of the most important equations in all of science, namely,
S = kB ln W
World’s Technological Capacity To Store And Communicate Entropic Information
A 2011 study in Science estimated the world’s technological capacity to store and communicate optimally compressed information normalized on the most effective compression algorithms available in the year 2007, therefore estimating the entropy of the technologically available sources. The author’s estimate that human kind’s technological capacity to store information grew from 2.6 exabytes in 1986 to 295 exabytes in 2007. The world’s technological capacity to receive information through one-way broadcast networks was 432 exabytes of information in 1986, to 1.9 zettabytes in 2007. The world’s effective capacity to exchange information through two-way telecommunication networks was 281 petabytes of information in 1986, to 65 exabytes in 2007.
You May Like: Beth Thomas Married
Entropy And Adiabatic Accessibility
A definition of entropy based entirely on the relation of adiabatic accessibility between equilibrium states was given by E.H.Lieb and J. Yngvason in 1999. This approach has several predecessors, including the pioneering work of from 1909 and the monograph by R. Giles. In the setting of Lieb and Yngvason one starts by picking, for a unit amount of the substance under consideration, two reference states X
- }\sum _p_\,\log \,p_,}
i.e. in such a basis the density matrix is diagonal.
Von Neumann established a rigorous mathematical framework for quantum mechanics with his work Mathematische Grundlagen der Quantenmechanik. He provided in this work a theory of measurement, where the usual notion of wave function collapse is described as an irreversible process . Using this concept, in conjunction with the density matrix he extended the classical concept of entropy into the quantum domain.
I thought of calling it “information”, but the word was overly used, so I decided to call it “uncertainty”. Von Neumann told me, “You should call it entropy, for two reasons. In the first place your uncertainty function has been used in statistical mechanics under that name, so it already has a name. In the second place, and more important, nobody knows what entropy really is, so in a debate you will always have the advantage.”
- . ^p\log p.}
is introduced into the system at a certain temperature T .
Entropy Equation And Calculation
There are multiple ways to calculate entropy, but the two most common equations are for reversible thermodynamic processes and isothermal processes.
Entropy of a Reversible Process
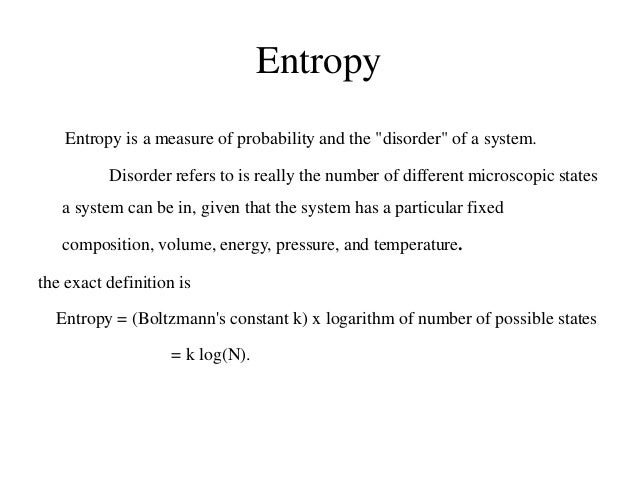
Certain assumptions are made when calculating the entropy of a reversible process. Probably the most important assumption is that each configuration within the process is equally probable . Given equal probability of outcomes, entropy equals Boltzmann’s constant multiplied by the natural logarithm of the number of possible states :
S = kB ln W
Boltzmann’s constant is 1.38065 × 1023 J/K.
Entropy of an Isothermal Process
Calculus may be used to find the integral of dQ/T from the initial state to final state, where Q is heat and T is the absolute temperature of a system.
Another way to state this is that the change in entropy equals the change in heat divided by the absolute temperature :
S= Q / T
Entropy and Internal Energy
In physical chemistry and thermodynamics, one of the most useful equations relates entropy to the internal energy of a system:
dU=T dS – p dV
Here, the change in internal energy dU equals absolute temperature T multiplied by the change in entropy minus external pressure p and the change in volume V.
You May Like: What Does Denominator Mean In Math
Entropy And The Search For Extraterrestrial Life
In 1964, James Lovelock was among a group of scientists requested by NASA to make a theoretical life-detection system to look for life on Mars during the upcoming space mission. When thinking about this problem, Lovelock wondered “how can we be sure that Martian life, if any, will reveal itself to tests based on Earths lifestyle? To Lovelock, the basic question was “What is life, and how should it be recognized? When speaking about this issue with some of his colleagues at the Jet Propulsion Laboratory, he was asked what he would do to look for life on Mars. To this, Lovelock replied “Id look for an entropy reduction, since this must be a general characteristic of life.”
The Second Law Of Thermodynamics What’snew
Morowitz The use of thermodynamics in biology has a long history rich in confusion.
Sometimes people say that life violates the second law of thermodynamics. This is not the case we know of nothing in the universe that violates that law. So why do people say that life violates the second law of thermodynamics? What is the second law of thermodynamics?
The second law is a straightforward law of physics with the consequence that, in a closed system, you can’t finish any real physical process with as much useful energy as you had to start with some is always wasted. This means that a perpetual motion machine is impossible. The second law was formulated after nineteenth century engineers noticed that heat cannot pass from a colder body to a warmer body by itself.
According to philosopher of science Thomas Kuhn, the second law was first put into words by two scientists, Rudolph Clausius and William Thomson , using different examples, in 1850-51 . American quantum physicist Richard P. Feynman, however, says the French physicist Sadi Carnot discovered the second law 25 years earlier . That would have been before the first law, conservation of energy, was discovered! In any case, modern scientists completely agree about the above principles.
You May Like: Holt Geometry Textbook Answers
Entropy Change And Calculations
During entropy change, a process is defined as the amount of heat emitted or absorbed isothermally and reversibly divided by the absolute temperature. The entropy formula is given as
S = qrev,iso/T
If we add the same quantity of heat at a higher temperature and lower temperature, randomness will be maximum at a lower temperature. Hence, it suggests that temperature is inversely proportional to entropy.
Total entropy change, Stotal =Ssurroundings+Ssystem
Total entropy change is equal to the sum of entropy change of system and surroundings.
If the system loses an amount of heat q at a temperature T1, which is received by surroundings at a temperature T2.
So, Stotal can be calculated
Ssystem=-q/T1
If Stotal is positive, the process is spontaneous.
If Stotal is negative, the process is non-spontaneous.
If Stotal is zero, the process is at equilibrium.
Points To Remember
|
Entropy change during the isothermal reversible expansion of an ideal gas
S = qrev,iso/T
According to the first law of thermodynamics,
U=q+w
For the isothermal expansion of an ideal gas, U = 0
qrev = -wrev = nRTln
S = nRln
Connecting Caliber To Other Thermodynamic Quantities
Despite the seeming advantages of caliber approaches over entropy to describe nonequilibrium dynamical systems, there are still some advantages of using entropy for certain problems. One of the major advantages to entropy methods is the strong fundamental connection between entropy and other thermodynamic metrics such as heat, free energy, work, and efficiency. The relationship between these metrics and caliber is less well-defined in the existing literature and offers an area rich for further research.
You May Like: Is Paris Jackson Blood Related To Michael
Entropy And Heat Death Of The Universe
Some scientists predict the entropy of the universe will increase to the point where the randomness creates a system incapable of useful work. When only thermal energy remains, the universe would be said to have died of heat death.
However, other scientists dispute the theory of heat death. Some say the universe as a system moves further away from entropy even as areas within it increase in entropy. Others consider the universe as part of a larger system. Still others say the possible states do not have equal likelihood, so ordinary equations to calculate entropy do not hold valid.
Extracting Energy From The Environment
Although organisms employ a wide range of specific strategies to obtain the energy they need to live and reproduce, they can generally be divided into two categories: organisms are either , whose energy source is sunlight, or chemotrophs, whose energy source is chemical compounds, usually obtained by consuming or breaking down other organisms. Phototrophs, such as plants, algae, and photosynthetic bacteria, use the radiant energy of the sun directly, converting water and carbon dioxide to energy-rich organic compounds, whereas chemotrophs, such as animals, fungi, and many nonphotosynthetic bacteria, obtain energy-rich organic compounds from their environment. Regardless of the nature of their energy and carbon sources, all organisms use oxidationreduction, or redox, reactions to drive the synthesis of complex biomolecules. Organisms that can use only O2 as the oxidant are aerobic organisms that cannot survive in the absence of O2. Many organisms that use other oxidants or oxidized organic compounds can live only in the absence of O2, which is a deadly poison for them such species are called anaerobic organisms.
Equation 18.49
This reaction is not a spontaneous process as written, so energy from sunlight is used to drive the reaction. Photosynthesis is critical to life on Earth it produces all the oxygen in our atmosphere.
Equation 18.50
Also Check: How Do Noise Canceling Headphones Work Physics