What Is Heat Capacity
When heat is absorbed by a body, the temperature of the body increases. And when heat is lost, the temperature decreases. The temperature of an object is the measure of the total kinetic energy of the particles that make up that object. So when heat is absorbed by an object this heat gets translated into the kinetic energy of the particles and as a result the temperature increases. Thus, the change in temperature is proportional to the heat transfer.
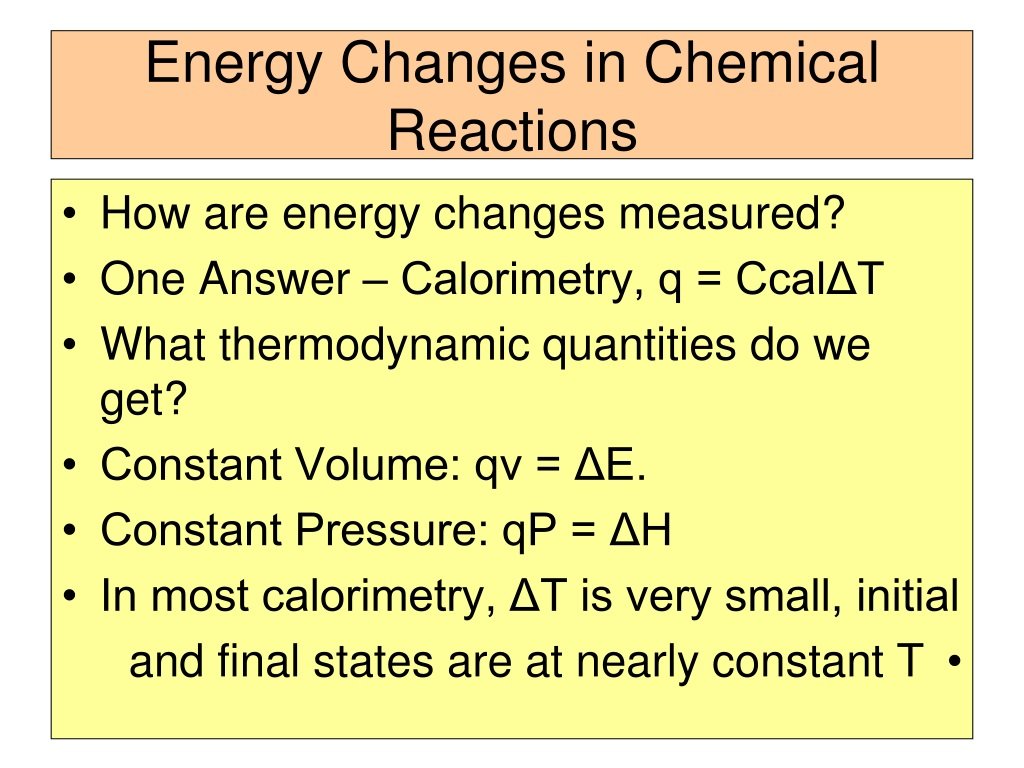
The formula q = n C T represents the heat q required to bring about a T difference in temperature of one mole of any matter. The constant C here is called the molar heat capacity of the body. Thus, the molar heat capacity of any substance is defined as the amount of heat energy required to change the temperature of 1 mole of that substance by 1 unit. It depends on the nature, size, and composition of the system.
In this article, we will discuss two types of molar heat capacity CP and CV and derive a relationship between Cp and Cv.
What Is Not A State Function
State functions depend only on the state of the system, not on the path used to get to that state. Heat and work are not state functions. Work cant be a state function because it is proportional to the distance an object is moved, which depends on the path used to go from the initial to the final state.
Enthalpy As A Composite Function
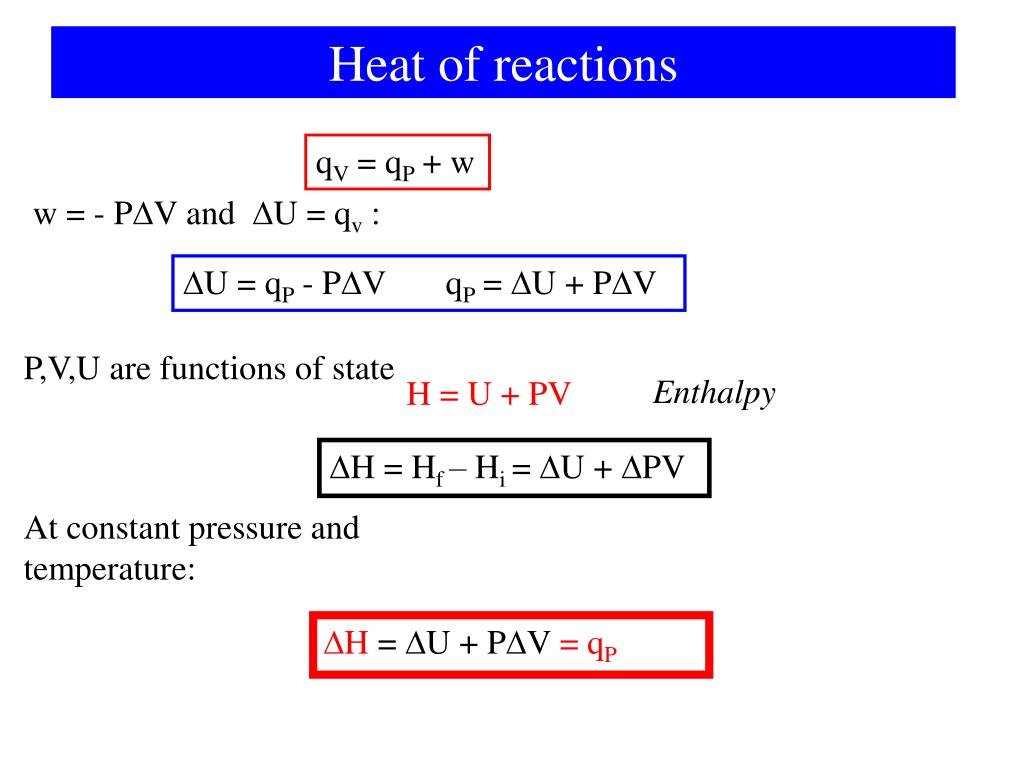
To further understand the relationship between heat flow ) and the resulting change in internal energy ), we can look at two sets of limiting conditions: reactions that occur at constant volume and reactions that occur at constant pressure. We will assume that PV work is the only kind of work possible for the system, so we can substitute its definition into the first Law of Thermodynamics ) to obtain the following:
where the subscripts have been deleted.
If the reaction occurs in a closed vessel, the volume of the system is fixed, and V is zero. Under these conditions, the heat flow must equal U:
At constant volume, no \ work can be done, and the change in the internal energy of the system is equal to the amount of heat transferred from the system to the surroundings or vice versa.
Many chemical reactions are not, however, carried out in sealed containers at constant volume but in open containers at a more or less constant pressure of about 1 atm. The heat flow under these conditions is given the symbol \ to indicate constant pressure. Replacing q in Equation \ by \ and rearranging to solve for qp,
Thus, at constant pressure, the heat flow for any process is equal to the change in the internal energy of the system plus the PV work done.
Because conditions of constant pressure are so important in chemistry, a new state function called enthalpy is defined as
Read Also: What Is An Open Sentence In Math
Difference Between Q And K And What Is Activity A
Therefore, by comparing Q and K, we can determine the direction of a reaction. Chemical activity of a species is a measure of the ‘effective concentration’ of a species. If the substance is a pure solid or liquid then a=1. When treating solutions or gases as ideal then the concentration, , is equal to the effective concentration, a.
What Is Qp Qv

Relation b/w heat of reaction at constant pressure and constant volume Qp = qv + AngRT where qp is the heat at constant pressure qy is the heat at constant volume Ang is the difference between the number of moles of the gaseous products and those of the gaseous reactants. > Chemistry. > Thermodynamics. > Enthalpy.
Read Also: Holt Mcdougal Geometry Worksheet Answers
Second Law Of Thermodynamics
The second law states that there exists a useful state variable called entropy. The change in entropy is equal to the heat transfer divided by the temperature . delta S = / T. For a given physical process, the entropy of the system and the environment will remain a constant if the process can be reversed.
What Is Pressure Volume Work
Pressurevolume work is the work that is done by the compression or expansion of a fluid. Whenever there is a change in volume and external pressure remains constant, pressurevolume work is taking place. … Because pressure is constant, the work done is PV . Recall that the formula for work is W=Fd.
Also Check: Edgenuity Health Unit Test Answers
Calculating The Reaction Quotient Q
The expression for the reaction quotient, Q, looks like that used tocalculate an equilibrium constant but Q can be calculated for any set ofconditions, not just for equilibrium. Q can be used to determine which direction a reaction will shift to reach equilibrium. If K > Q, a reaction will proceed forward, converting reactants into products.
What Is Q Mc T Used For
Q=mcT Q = mc T , where Q is the symbol for heat transfer, m is the mass of the substance, and T is the change in temperature. The symbol c stands for specific heat and depends on the material and phase. The specific heat is the amount of heat necessary to change the temperature of 1.00 kg of mass by 1.00ºC.
Recommended Reading: Holt Geometry Chapter 7
How Do I Calculate Delta H
Use the formula H = m x s x T to solve. Once you have m, the mass of your reactants, s, the specific heat of your product, and T, the temperature change from your reaction, you are prepared to find the enthalpy of reaction. Simply plug your values into the formula H = m x s x T and multiply to solve.
Is Qp Equal To Qv
qv is heat at a constant volume while qp is heat at a constant pressure. qp is usually measured with a calorimeter while qv can be measured with a bomb calorimeter. qp also equals delta H or enthalpy where pressure is constant. At a constant volume, deltaU = qv, since no work of expansion is being done on the system.
Also Check: Holt Geometry Chapter 7 Test Form A Answers
Cation Exchange Capacity And Base Saturation
Cation exchange capacity gives an insight into the fertility and nutrient retention capacity of soil. Certain soil minerals, such as clay, particularly in combination with organic matter, possess a number of electrically charged sites, which can attract and hold oppositely charged ions. The negatively charged sites make up the CEC, the ability to hold H+, Ca2+, Mg2+, Na+, and NH4+, etc., and the positively charged sites, which hold OH, SO4=, NO3, PO4=, etc., make up the anion exchange capacity. Ions held at these sites can be exchanged with others of similar charge. CEC is an important index of nutrient status because exchangeable cations are the most important source of immediately available plant nutrients. Over a course of time, CEC was found to increase, due to increases in the nutrient storage capacity of mine spoils.
Base saturation is an important parameter that influences tree growth in mine soils . BS and CEC depends on the rock type/parent material of the mine spoil . In fresh mine spoils, BS will be higher but once exposed to natural weathering process, they leach slowly. However, over a course of time as the mine spoil get reclaimed, the BS increases due to the accumulation of clay sized weathering products .
Colin McPhee, … Izaskun Zubizarreta, in, 2015
Which Of The Following Property Is Path Function
From all the above given thermodynamic quantities, work is the only property which depends on the path followed by the system i.e. on the initial and the final states of the system. On the contrary, the rest all others i.e. internal energy, enthalpy and entropy are path independent. Hence, option is correct.
You May Like: What Does Ppt Mean In Chemistry
The Relationship Between Cp And Cv For An Ideal Gas
From the equation q = n C T, we can say:
At constant pressure P, we have
qP = n CPT
This value is equal to the change in enthalpy, that is,
qP = n CPT = H
Similarly, at constant volume V, we have
qV = n CVT
This value is equal to the change in internal energy, that is,
qV = n CVT = U
We know that for one mole of an ideal gas,
H = U + = U + = U + R T
Therefore, H = U + R T
Substituting the values of H and U from above in the former equation,
CPT = CVT + R TCP = CV + R
To learn more about thermodynamics and heat transfer, download BYJUS The Learning App.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
What Is Qv In Chemistry
4.7/5Qv
In this regard, what is the relationship between QP and QV?
qp vs. qv. I want to make sure that my understanding of heat at constant temperature versus constant pressure is correct. Thus, at constant pressure: Up=qp-PV at constant volume: Uv=qv and, finally, at constant pressure: H=U+PV.
Additionally, what does hrxn mean? Hrxn, or the change in enthalpy of a reaction, has the same value of H as in a thermochemical equation, but is in units of kJ/mol being that it is the enthalpy change per moles of any particular substance in the equation.
Consequently, is QP a state function?
qp. Work is a state property as it is directly proportional to the object’s distance moved against the opposing force and this distance depends on the path taken. Since deltaU = W + q, and work is a state function, it seems that the heat given off must be dependent on the path as well.
How do you define enthalpy?
Enthalpy is a thermodynamic property of a system. It is the sum of the internal energy added to the product of the pressure and volume of the system. It reflects the capacity to do non-mechanical work and the capacity to release heat. Enthalpy is denoted as H specific enthalpy denoted as h.
Also Check: What Is Causation In Psychology
What Does Q Represent In Chemistry
In Chemistry, Q stands for the reaction quotient. The reaction quotient Q, plays a major role in a chemical reaction. It can be used to determine the value of the products and reactants at any point in a chemical reaction. It is also used to determine the direction a chemical reaction is most likely going to follow, if the value of the products …
What Does Q Or Q Mean In Chemistry
The reaction quotient measures the relative amounts of products and reactants present during a reaction at a particular point in time. The reaction quotient aids in figuring out which direction a reaction is likely to proceed, given either the pressures or the concentrations of the reactants and the products.
Read Also: What Influence Did Geography Play In The Development Of Greek Society
Can You Go Over Q = M * C * Deltat + Example
The specific heat capacity, or simply specific heat of a substance is the amount of heat energy required to raise the temperature of one gram of the substance by one degree Celsius. Heat energy is usually measured in Joules or calories . The variables in the equation q = mCDeltaT mean the following: ” let:” q=”heat energy gained or lost by a substance” m=”mass ” C …
Q Vs K To Predict Direction Of Reaction Chemistry Tutorial
As the reaction proceeds in the forward direction towards equilibrium, the value of the mass-action expression, Q, is increasing. At equilibrium, around t=4, the value of the mass-action expression , Q, becomes constant and is equal to the value of the equilibrium constant, K, for this reaction at this temperature. At equilibrium: Q = 6.09 = K.
You May Like: What Happened To Beth Thomas Biological Father
Standard Enthalpy Of Reaction
The enthalpy of reaction is defined as the internal energy of the reaction system, plus the product of pressure and volume. It is given by:
H=U+PV
By adding the PV term, it becomes possible to measure a change in energy within a chemical system, even when that system does work on its surroundings. Most often, we are interested in the change in enthalpy of a given reaction, which can be expressed as follows:
\Delta H = \Delta U +P\Delta V
When you run a chemical reaction in a laboratory, the reaction occurs at constant pressure, because the atmospheric pressure around us is relatively constant. We will examine the change in enthalpy for a reaction at constant pressure, in order to see why enthalpy is such a useful concept for chemists.
What Are The Limitations Of First Law Of Thermodynamics
The limitation of the first law of thermodynamics is that it does not say anything about the direction of flow of heat. It does not say anything whether the process is a spontaneous process or not. The reverse process is not possible. In actual practice, the heat doesnt convert completely into work.
Recommended Reading: Fsa Algebra 1 Eoc Practice Test Answers
Determining The Heat Of Reaction
The amount of heat that the system gives up to its surroundings so that it can return to its initial temperature is the heat of reaction. The heat of reaction is just the negative of the thermal energy gained by the calorimeter and its contents ) through the combustion reaction.
where
If the constant volume calorimeter is set up the same way as before, then the heat capacity of the calorimeter can be measured using the following formula:
Heat capacity is defined as the amount of heat needed to increase the temperature of the entire calorimeter by 1 °C. The equation above can also be used to calculate \ from \ calculated by Equation \ref. The heat capacity of the calorimeter can be determined by conducting an experiment.
Example \: Heat of Combustion
1.150 g of sucrose goes through combustion in a bomb calorimeter. If the temperature rose from 23.42 °C to 27.64 °C and the heat capacity of the calorimeter is 4.90 kJ/°C, then determine the heat of combustion of sucrose, \ ).
Solution
- mass of \: 1.150 g
- \: 23.42°C
- Heat Capacity of Calorimeter: 4.90 kJ/°C
Using Equation \ref to calculate \:
\ & = \ kJ = 20.7\ kJ \end\]
Plug into Equation \ref:
\ & = -20.7 \ kJ \ \end\]
But the question asks for kJ/mol \, so this needs to be converted:
\ & = \dfracH_O_} \end\]
Convert to per Mole \:
\ & = \dfrac} \end\]
“Ice Calorimeter”
What Is Qp In Chemistry
Abstract The reaction quotient Q can be expressed in partial pressures as QP or in mole fractions as Qx.Feb 13, 2014
What is QP in thermodynamics?, Relation b/w heat of reaction at constant pressure and constant volume Qp = qv + AngRT where qp is the heat at constant pressure qy is the heat at constant volume Ang is the difference between the number of moles of the gaseous products and those of the gaseous reactants.
Furthermore, What does QP mean in enthalpy?, The q refers to heat but qp is enthalpy because it describes heat under a constant pressure. Enthalpy can be endothermic or exothermic, meaning qp can be positive or negative as well.Jan 25, 2019
Finally, What is the difference between QV and QP?, Qv is constant volume, Qp is constant pressure. Lavelle used Qv in the example of the bomb calorimeter and Qp in the coffee cup calorimeter. Qv: heat absorbed or released at constant volume. Qp: heat absorbed or released at constant pressure and gives enthalpy values.Jan 12, 2018
Recommended Reading: Who Is The Mother Of Paris Jackson
The Reaction Quotient Q
The reaction quotient is a measure of the relative amounts of products and reactants present in a reaction at a given time. For reversible reaction , where , , , and are the stoichiometric coefficients for the balanced reaction, we can calculate using the following equation: This expression might look awfully familiar, because is a concept that …
Qv And Qp Relationship
Is there any relationship between q at constant volume and q at constant pressure that I could derive with just the reaction equation, and the enthalpy of formation and Cp of the products and reactants?
I am aware that q at constant pressure is enthalpy of formation of products – enthalpy of formation of reactants.
- Chet MillerSep 28, 2020 at 16:27
- $\begingroup$I had not assumed that but I believe we would have to as one of our state variables.$\endgroup$
Let us assume that the initial and final temperatures in both the isobaric and isochoric processes are same. We will limit this discussion to ideal gases only. To calculate heat transfered we will use the expression $$q = nC\Delta T$$ Here C is molar heat capacity of the gas. For isobaric process, this will be $$q_p = nC_p\Delta T$$ For the isochoric process $$q_v=nC_v\Delta T$$$\Delta T$ for both these processes same. Substracting these two equations we get $$q_p – q_v = n\Delta T$$ The value of $C_p – C_v$ is $R$. Therefore $$q_p – q_v = nR\Delta T$$
Also Check: Geometry Dash Toe2
What Does Qv Stand For
What does QV mean? This page is about the various possible meanings of the acronym, abbreviation, shorthand or slang term: QV.
Filter by:
Popularity rank for the QV initials by frequency of use:
Couldn’t find the full form or full meaning of QV?
Maybe you were looking for one of these abbreviations:
Discuss these QV abbreviations with the community:
Report Comment
We’re doing our best to make sure our content is useful, accurate and safe.If by any chance you spot an inappropriate comment while navigating through our website please use this form to let us know, and we’ll take care of it shortly.