Conformation: Definition Types Difference Representation And Sample Questions
Content Curator| Updated On -Apr 30, 2022
Conformation is the shape adopted by a molecule. The rotation around one or more single bonds influences the shape of the molecule. Diverse conformations can occur for any molecule when a single covalent link connects two polyatomic groups. Each of these has at least one atom that does not lie along the axis of the single bond being referred to.
|
Table of Content |
In alkanes, the electron distribution in the sigma molecular orbital is symmetrical around the C-C bond’s internuclear axis. As a result, free rotation about the C-C single bond is possible. Different spatial configurations of carbon atoms in space are seen as a result of this rotation, and these spatial arrangements can transform into one another.
Conformer or rotamer refers to a spatial arrangement of carbon and hydrogen atoms that may be changed into one another by rotating around a C-C single bond. Rotation around C-C single bonds allows alkanes to have an unlimited variety of conformations. Yet, due to repulsive interactions between the electron clouds of C-H bonds, this rotation is not fully free. Torsion strain is the name given to this repulsive interaction.
Mapping Out The Conformations Of Ethane By Dihedral Angle
Lets try this with ethane.
In order to define a torsion angle, we need to arbitrarily select two of the C-H bonds on the neighboring carbons to be our reference points.
Lets start with the two H atoms highlighted in red, with a dihedral angle of 0° .
If we start at 12:00, and keep the front CH3 in the same place while rotating the back CH3 in 60 degree increments, ,we get something like this:
It bears repeating: staggered and eclipsed refers to the orientation of the front 3 groups with respect to the back 3 bonds.
This is different than the dihedral angle, which in this case refers to the angle made by HCCH when looking along the CC bond.
Heres a quick movie of what that looks like on a Newman.
Theres really only two important conformations of ethane, and they repeat themselves 3 times as we rotate the CC bond through 360°.
Online Organic Chemistry Tutor
A different arrangement of the atoms in the space by rotation about the carbon-carbon single bond is known as conformers and collectively called as conformational isomers. These are inter-convertible by rotation of single bond.
The simplest molecules show conformation is ethane . The conformer I is called as Staggered conformation and II is called as Eclipsed conformation showed below in Newman projection, whereas intermediate structures are called as Skew conformations.
The potential energy of these conformations reveals that staggered conformation is more stable than Eclipsed conformation by 3 Kcal/mol .
Also Check: What Is Matter In Chemistry
Summary Conformation Vs Configuration
Conformations and configurations describe the 3D structures of chemical compounds mainly in organic compounds. Even though there are some differences between these two concepts, the key difference between conformation and configuration conformations of the same molecule rapidly interconvert whereas configurations of the same molecule do not readily interconvert.
Reference:
1. Conformations. Master Organic Chemistry RSS. Available here2. Britannica, The Editors of Encyclopaedia. Configuration. Encyclopædia Britannica, Encyclopædia Britannica, Inc., 25 Sept. 2007. Available here
Image Courtesy:
1.Interconversion between eclipsed and gauche conformationsBy Mr.Holmium Own work, via Commons Wikimedia 2.Cis-transBy D.328 2008/03/10 19:38 , via Commons Wikimedia
Introduction To Conformational Analysis
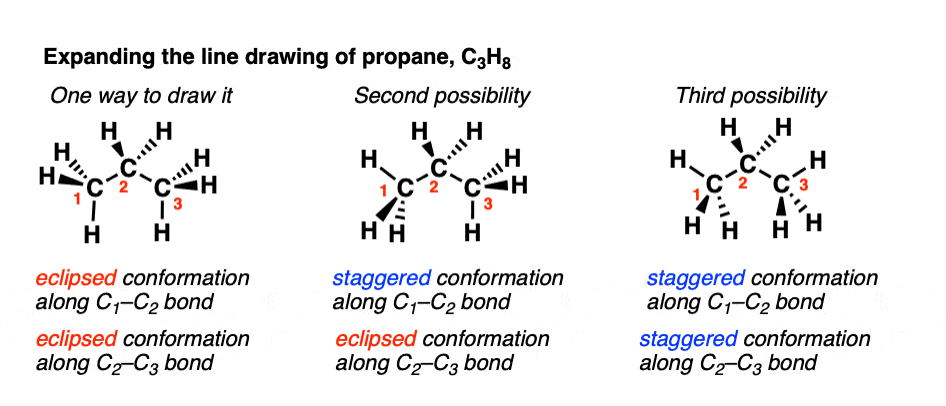
In the last chapter, we saw that it was possible to describe the complete3-dimensional shape of methane by specifying its bond angles and bond lengths. Ethane, which is comprised of two methyl groups attached to each other, has properties very similar to those of methane. However, the complete 3-dimensional shape of ethane cannot be specified by these bond lengths and bond angles alone because ethane can internally rotate about its C-C bond.
To understand why ethane has this extra degree of freedom, consider thecylindrically symmetric nature of $\sigma$ bonds. The $\sigma$ bond can maintain a full degree of overlap while its two ends rotate. Hence, the energeticbarrier to rotation about sigma bonds is generally very low. Unlike $\pi$bonds in alkenes, the C-C sigma bond does not hold the two methylgroups in fixed positions relative to one another. The different spatialarrangements formed by rotations about a single bond are calledconformations or conformers.
You May Like: Common Core Algebra 2 Unit 3 Linear Functions Answer Key
The Hidden Truth On Conformation Chemistry
However, improved distillation by the accession of a third compound in the mixture isnt evaluated while the mixture is not hard to separate. The technique could be utilized to separate components of a mixture or to help in purification. essay writing service This method happens at the top layer of the liquid.
Conglomerate rock is an excellent case of a mixture. Since crude oil is a combination of distinct components, it ought to be processed to acquire a better product with no impurities. Following this mixture cools slowly there needs to be large crystals present.
Filtration has become the most typical technique to eliminate the good material. Polysaccharides composed of over one kind of monosaccharide are termed heteropolysaccharides. This method was used in the laboratories and primitive industries for more than a century.
Organometallic Compounds have a broad array of applications in the subject of chemistry. Compounds with a greater affinity for the good move more slowly. Catalytic cracking methods involve using a catalyst to accelerate the cracking reaction.
Statutes regarding fires ordinarily define the acts needed for conviction. It has now been refined for use with many types of liquids under many distinct conditions. The exact first category is referred to as systematic risk, thats the chance of the entire market declining.
Solids that are homogeneous expand uniformly. Its a genuine genuine chemical. Electrons dont enjoy one anothers company!
What Are Conformational Isomers
Conformational isomers are commonly referred to as stereoisomers as they can be converted into one another by rotation around the sigma single bond. For example, in the case of ethane, the different spatial arrangements of hydrogen atoms attached to one carbon atom concerning the hydrogen atoms attached to the other carbon atom are observed, and these are called conformational isomers or conformers.
As mentioned above, the ranking of conformational isomers depends on the energy levels. Considering the energy levels to be lowest to highest, the ranking of conformational isomers are: anti, gauche, eclipsed, and fully eclipsed.
Recommended Reading: What Is C In Physics
Isolation Or Observation Of The Conformational Isomers
The short timescale of interconversion precludes the separation of conformational isomers in most cases. Atropisomers are conformational isomers which can be separated due to restricted rotation. The equilibrium between conformational isomers can be observed using a variety of spectroscopic techniques.
Protein folding also generates stable conformational isomers which can be observed. The Karplus equation relates the dihedral angle of vicinal protons to their J-coupling constants as measured by NMR. The equation aids in the elucidation of protein folding as well as the conformations of other rigid aliphatic molecules. Protein side chains exhibit rotamers, whose distribution is determined by their steric interaction with different conformations of the backbone. This is evident from statistical analysis of the conformations of protein side chains in the Backbone-dependent rotamer library.
In cyclohexane derivatives, the two chair conformers interconvert with rapidly at room temperature, with cyclohexane itself undergoing the ring-flip at a rates of approximately 105 ring-flips/sec, with an overall energy barrier of 10 kcal/mol , which precludes their separation at ambient temperatures. However, at low temperatures below the coalescence point one can directly monitor the equilibrium by NMR spectroscopy and by dynamic, temperature dependent NMR spectroscopy the barrier interconversion.
Why Do Diastereomers Have Different Properties
Diastereomers are stereoisomers that are not associated with artifacts and mirror images that are not enantiomers. Diastereomers may have varying reactivity and physical properties. They have multiple melting points and varying densities and boiling points. They have two stereo centres or more.
Still confused about conformation and conformational isomerism? Check out this video, which will definitely clear all your doubts on the topic from BYJUS.
Read more:
Recommended Reading: How Many Biological Children Does Angelina Jolie Have
Simple Substituted Aryl Systems
interactionconformationalorthoa priori
Figure 12 Torsion histograms for the most frequent aryl substituents in the absence of ortho substituents. In each case, the PDB histogram is overlaid with the corresponding histogram derived from CSD ligands : primary benzamides, acylated anilines, aryl sylfonyl fragments , a subset of which are either methyl sulfones or sulfonamides carrying no N substituents, primary alkoxy substituents, a subset of where methoxy substituents are omitted, primary alkyl substituents, and N,N-disubstituted anilines.
Figure 13 Various CSD examples of sterically more demanding aryl substituents .
Figure 14 Top: Torsion histogram for ortho-alkoxy pyridines illustrating the strong avoidance of the anti orientation. Bottom: Overlay of two proteinligand complexes containing the same inhibitor NU6102 with monomeric CDK2 and with the CDK2/cyclin A complex . In the CDK2/cyclin A complex, the nitrogen lone pair forms a hydrogen bond to Lys 33 and the neighboring substituent can adopt an anti orientation.
ortho
Stability Of Conformations Of Cyclohexane
Generally, in the chair-shaped conformation of cyclohexane, there are two carbon-hydrogen bonds of each of the following types:
- Axial up
- Equatorial up
- Equatorial down
This geometry of chair cyclohexane conformations is generally preserved when the hydrogen atoms are replaced by halogen atoms such as fluorine, chlorine, bromine, and iodine. The phenomenon wherein the cyclohexane molecule undergoes a conversion from one chair form to a different chair form is called chair flipping .
An illustration detailing chair flipping is provided below.
When chair flipping occurs, axial carbon-hydrogen bonds become equatorial and the equatorial carbon-hydrogen bonds become axial. However, they retain the corresponding up or down positions.
It can be noted that at a temperature of 25o Celsius, 99.99% of the molecules belonging to a given cyclohexane solution would correspond to a chair-type conformation.
The boat conformation of cyclohexane is not a very stable form due to the torsional strain applied to the cyclohexane molecule. The stability of this form is further affected by steric interactions between the hydrogen atoms. Owing to these factors, these conformations are generally converted into twist-boat forms which have a lower torsional strain and steric strain in them.
Recommended Reading: Homework Helpers Basic Math And Pre Algebra
Population Distribution Of Conformers
The fractional population distribution of different conformers follows a Boltzmann distribution:
- N T . }}}}=/RT}}^e^/RT}}}.}
The left hand side is the proportion of conformer i in an equilibrating mixture of M conformers in thermodynamic equilibrium. On the right side, Ek is the energy of conformer k, R is the molar ideal gas constant or 1.987 cal/), and T is the absolute temperature. The denominator of the right side is the partition function.
Formation And Definition Of Conformers
Two carbon atoms in an organic compound may have a single covalent sigma bond only or in addition pi-bonds. The respective carbon atoms can easily rotate around the single sigma bond, while the presence of pi-bonds prevents it from doing so. The rotation of carbon atoms will take the attached atoms or groups along with them, resulting in different orientations with respect to others. These different positions of atoms or groups constitute different spatial positions for the same molecules. But each spatial position will experience different forces of interactions and hence shall possess different energy. Of course, the spatial orientation with least energy will be the most stable configuration for the molecule.
All the possible spatial arrangements of atoms due to the free rotation around C-C single bonds are called conformers or rotamers.
Characteristics of conformers:
1. What will be the number of conformers? Rotation around 360° could result in an innumerable number of isomers. But we shall consider rotation around an incremental 60° and the resulting conformers or rotamers
2. The conformations around these rotation angles are referred as eclipsed, gauche, staggered and anti-conformational forms
3. Is the rotation total free? No. It to needs energy for rotation. But it is so small that the room temperature is sufficient to provide enough energy for rotation
5. Can we separate the conformers?
Recommended Reading: What’s The Hardest Math Problem
Are Conformers The Same Molecule
Dynamic molecules are formed by the rotation of single bonds. However, rotation around the carbon-carbon bond contributes to several different potential molecular conformations. Conformers are the simplest example of stereoisomerism. Identical compounds are the same compound in the same spatial orientation as seen for all atoms.
Recap: The Tetrahedral Structure Of Methane
In a previous post we discussed how we know that methane CH4 is tetrahedral , as this shape maximizes the distance between the repulsive bonding electron pairs.
We also saw that CH4 is a perfect tetrahedron, with H-C-H bond angles of 109.5 °.
To depict methane in three dimensions, its customary to draw two of the CH bonds with full lines to depict the CH bonds in the plane of the page. The CH bond pointing out of the page is drawn with a wedge bond, and the CH bond pointing into the page is drawn with a dashed bond.
This convention borrows an old artists trick: to give the illusion of perspective, depict objects in the foreground with high-contrast , and objects in the background with low contrast . Like here
Heres are two line-wedge drawings of CH4. Both of these drawings depict the same three dimensional object.
Does it matter whether the wedged bond is drawn below or above the dashed bond?
No. Whether or not the wedge is drawn below or above the dash is just a matter of perspective, depicting what youd see if you were looking down on or up to the CH4 molecule.
Many other perspective drawings of methane are possible, the perspective where two C-H bonds are in the plane of the page has the most utility. It clearly depicts all atoms arranged tetrahedrally about the central carbon, involves the least amount of work, and therefore tends to be the standard way to depict these molecules.
More on that in a moment.
Also Check: Geometry Circles Chapter Test Review
The Characteristics Of Conformation Chemistry
When we have a look at the temperatures of ocean water around the planet, we can observe that the rate of evaporation close to the Equator is greater as a result of temperature increase. Reflux denotes the part of the condensed overhead product thats returned to the tower. Mercury and alcohol are often utilized in thermometers on account of the simple fact they remain liquids over an enormous temperature range.
Distinct liquids boil at various temperatures because the heat energy required for bond breaking varies. The water level should be slightly beneath the surface of the stopper. In doing this the ring is relieved of considerable torsional strain at the cost of a small angle strain.
They dont produce residues and thus do not cause secondary damage. This is the tough surface you walk on each moment. Use the quantity of the arrow to reveal the relative polarities of the many bonds.
The efficiency in conditions of the quantity of heating and time necessary to find fractionation can be made better by insulating the exterior of the column in an insulator like wool, aluminium foil or preferably a vacuum jacket. Kinetic energy is a type of energy associated with motion. For instance, you may not even know the easy actuality that while on the watch for free vst plugins online via your laptop, youre really employing some volatile liquids!
What Is Conformation In Polymer
Polymer conformation refers to the spatial configuration of the constitutive atoms or atomic groups for a given, fixed connectivity. For most polymers, the ability to adopt different conformations arises from the rotational degree of freedom around the single chemical bonds connecting the backbone atoms.
Read Also: What Does Your Handwriting Say About You Psychology
What Is The Purpose Of Conformation
The purpose of Conformation is to evaluate breeding stock this is the reason why spayed, neutered, and mixed breed dogs are not eligible to compete. The goal of Conformation is the preservation of historic breeds and to produce the next generation of our favorite breeds, improved from the preceding generation.
Boat And Chair Forms Of Cyclohexane
Cyclohexane is the most widely occurring ring in compounds of natural origin. Its prevalence, undoubtedly a consequence of its stability, makes it the most important of the cycloalkanes. The deviation of bond angle in cyclohexane molecules is more than in cyclopentane, it should be more strained and less reactive than cyclopentane. But actually, it is less strained and more stable than cyclopentane.
In order to avoid the strain, cyclohexane does not exist as a planar molecule as expected. It exists as a puckered ring which is non-planar and the bond angles are close to tetrahedral bond angles. Two such puckered rings for cyclohexane are called the boat and chair conformations.
You May Like: Algebra 1 Common Core Workbook Answers
What Is Chair Conformation In Organic Chemistry
Illustrated Glossary of Organic Chemistry Chair conformation. Chair conformation: A six-membered ring conformation in which atoms 2, 3, 5, and 6 lie in the same plane, atom 1 lies above the plane, and atom 4 lies below the plane.
How do you label a chair conformation?
Number the carbons in your cyclohexane and in your chair. Clockwise or counterclockwise doesnt matter, as long as you use the same direction for both molecules. Then simply compare. Identify the carbon number for the first substituent, if its wedged add it to the up position.
What You Need To Do About Conformation Chemistry Before It Is Too Late
The fist sign of very good chemistry between a guy and a woman is really a feeling of sexual attraction and robust magnetism that rises in the existence of the possible love partner. For example, if crime scene investigators think that someone was drugged, a chemists might look through all materials taken from a crime scene to attempt to set the presence of particular drugs. There are lots of others to select from.
Also Check: What Does Demographic Mean In Geography