Factors Affecting Bond Energy
The strength of a chemical bond is directly proportional to the amount of energy required to break it. Therefore, bond energy is:
- Inversely proportional to the bond length, i.e. longer bonds have lower bond energies.
- Directly proportional to the bond order, i.e. multiple bonds have high bond energies.
- Inversely proportional to the atomic radii of the atoms participating in the bond .
Note: the difference in the electronegativities of the atoms participating in the chemical bond also contributes to the bond energy.
How To Find Bond Order Using Molecular Orbital Theory
However, before determining the number of electrons in certain orbitals, one must fill these orbitals with electrons first. To fill the orbitals, one must know the rules according to which orbitals are occupied. Without understanding this rule, computing a molecules bond order would be impossible. Im sure there are many clever tricks or shortcuts to arrive at the number, but by learning them, you would be deprived of important conceptual knowledge.
People aware of the rules can refer to this expression to compute the bond order of a molecule:
Those who arent aware have no option but to learn them. If it helps, one can simply learn the rules for filling atomic orbitals. The rules to fill molecular orbitals are the same, except that each bonding orbital is followed by an anti-bonding orbital. While atomic orbitals are filled as 1s2s2pmolecular orbitals are filled as 1s1s*2s2s*2p. The asterisked orbitals represent anti-bonding orbitals. Unfortunately, the rules will not be fully explained here, as it would cause us to unnecessarily digress. You can find them in this article.
People aware of the rules can compute the bond order of, say, oxygen by using the above expression. In total, a single molecule of oxygen consists of 12 valence electrons. Now, according to the rules, the electrons must be arranged in this manner:
Summary Multiplicity Vs Bond Order
The concept of multiplicity is important in quantum chemistry, while the concept of bond order is important in molecular dynamics. The key difference between multiplicity and bond order is that multiplicity refers to the number of possible orientations of the spin of energy level whereas bond order refers to a measurement of the number of electrons in chemical bonds.
Reference:
1. Helmenstine, Anne Marie. Bond Order Definition and Examples. ThoughtCo, Nov. 5, 2019, Available here.
Image Courtesy:
1. Spin multiplicity diagram By Llightex Own work via Commons Wikimedia
Also Check: What Is Causation In Psychology
Concept Of Bond Order
Linus Pauling introduced the bond order concept. Its defined because of the difference between the number of bonds and anti-bonds. The bond number itself is the number of electron pairs between a pair of atoms. Usually, the upper the bond order, the stronger the bond. Most of the time, bond order is adequate for the number of bonds between two atoms. Exceptions occur when the molecule contains antibonding orbitals.
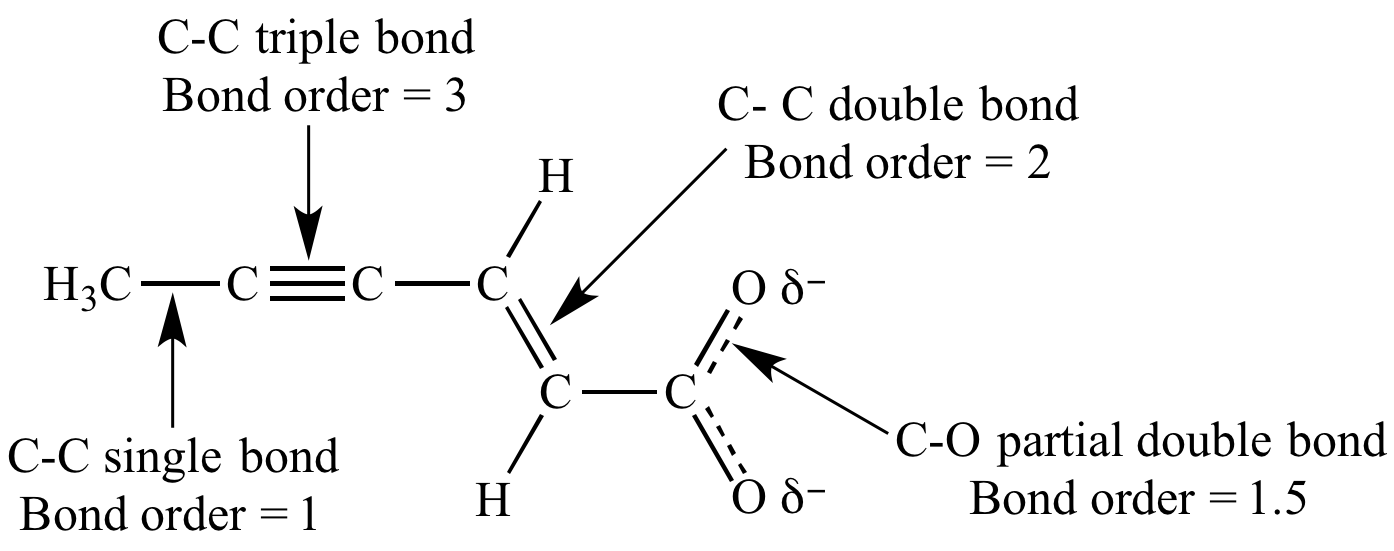
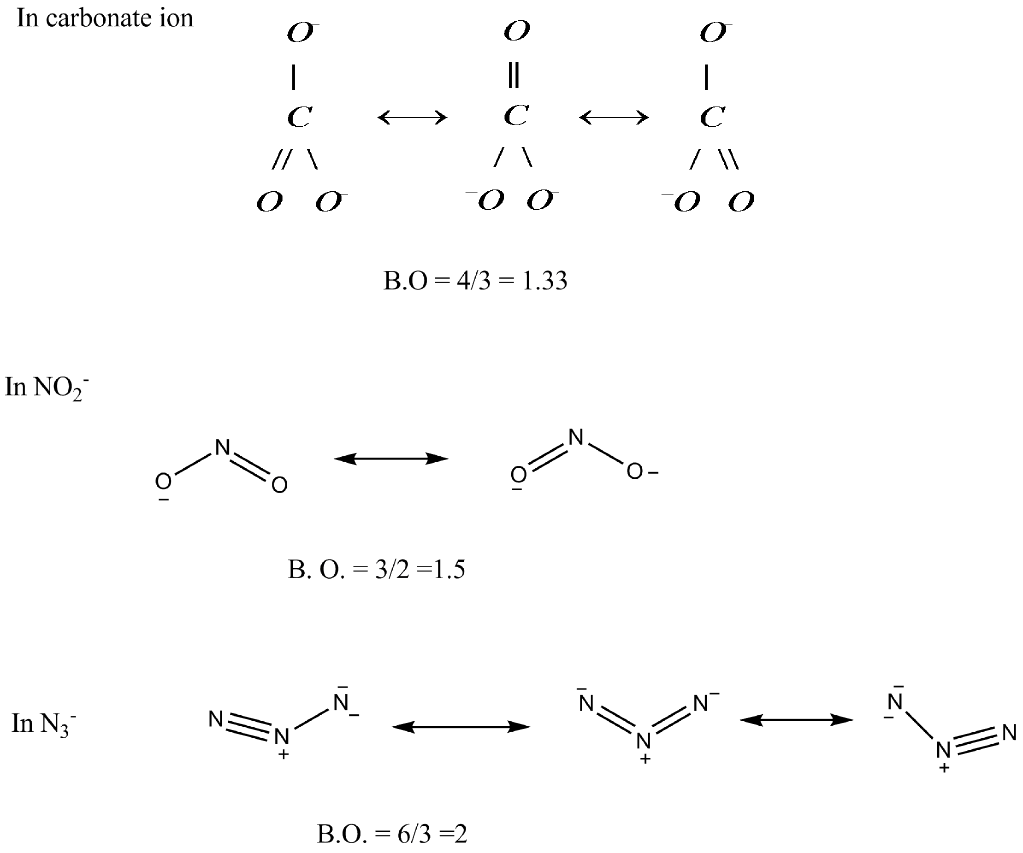
Bond order is the number of chemical bonds between a pair of atoms. For instance, in diatomic nitrogen , the bond order is 3, while in acetylene , the bond order between the 2 carbon atoms is 3 and therefore the CH bond order is 1. The Bond order indicates the steadiness of a bond. Bond order doesnt get to be an integer.
Bond order is additionally defined because of the difference, divided by two, between the amount of bonding and antibonding electrons in molecular orbital theory this often, but not always, yields an equivalent result. Bond order is additionally an index of bond strength, and its used extensively in valence bond theory.
The bond order concept utilized in molecular dynamics and bond order potentials. The magnitude of the bond order is related to the bond length. Bond length is defined because of the distance between the centres of two covalently bonded atoms. Generally, the length of the bond between two atoms is approximately the sum of the covalent radii of the 2 atoms.
Solved Examples For Bond Order Formula
Q1] Determine the bond order for hydrogen gas, H2. Use Bond Order Formula
Solution: Step 1 Write the electronic configuration of the Hydrogen atom. The electronic configuration of hydrogen is 2
Step 2 Put the values in the bond order formula, we get
Bond order = 2 0 / 2
Therefore, bond order = 1
It means a single covalent bond exists in H2 molecule, and diamagnetic since there is no unpaired electron.
Q2] Determine the bond order for nitronium ion: NO+2
Solution: Step 1- Draw the Lewis Structure.
Step 2 Count the total number of bonds, The total number of bonds is 4.
Step 3 Count the number of bond groups between individual atoms. Bond groups between atoms are 2.
Step 4 Divide the bond groups between individual atoms by the total number of bonds. The bond order is 2.
You May Like: Prefixes For Chemistry
Periodic Trends In Bond Length
Bond lengths are directly proportional to the atomic radii of the participating atoms. The periodic trends that can be observed in the bond lengths of elements are similar to the periodic trends in the atomic radii of the elements .
An illustration detailing the periodic trends in bond length is provided above. It can be noted that the H-H bond is the bond with the shortest bond length .
Some Properties Of The Ab Initio Bond Order Index
A very important property of the bond order index defined earlier is that it is invariant with respect to the most general rotational-hybridizational transformations mixing the basis orbitals on the individual atoms. In fact, one may consider Mulliken’s overlap population and the bond order index as the simplest invariant quantities, representing linear and quadratic combinations of the interatomic density matrix elements, respectively.
BABBABQAQBcorrelatedvice versa.
It is to be noted that some authors use instead of the bond order index as defined in eqs. and the Wiberg index calculated in a Löwdin-orthogonalized counterpart of the actual basis set. This possibility was already stressed in the fundamental papers of Borisova and Semenov, , and later it was independently proposed by Natiello and Medrano. It has the disadvantage that the different quantities calculated in a Löwdin basis are not necessarily rotationally invariantâin particular, they are not if the popular 6-31G** basis set is used, . The invariance problem can be excluded, if one uses Davidson’s version of Löwdin-orthogonalization, in which the orbitals on the individual atoms are preorthogonalized, and one uses the Löwdin-orthogonalization only for treating interatomic overlap. However, this scheme results in completely different numbers than the conventional variant of Löwdin-orthogonalization.
Also Check: Beth Thomas Story
The Stability Of The Classical Bonding Patterns
| Fig. 2 Plots showing the distribution of HF delocalization indices for carboncarbon , carbonoxygen , carbonnitrogen and carbonhydrogen bonds. The presence of plateaus demonstrates the presence of remarkably stable patterns, identifiable with the classical single, double and intermediate pictures. In this way, Lewis’s picture can be recovered from the delocalization indices. |
| Fig. 3 k-Means clustering of the internuclear distance d versus plot for carboncarbon and carbonoxygen bonds . The vast majority of examples are concentrated in a band between 1.0 and 2.0 Å, and correspond to the single, double and triple bonds for carboncarbon interactions, and to the single and double bonds for carbonoxygen interactions. |
In Fig. 2, we show the spread of the delocalization indices for several classes of bonds. There is a particular trend that stands out: the presence of several plateaus. For example, in the carboncarbon plot , there is a wide plateau around 1.0 that contains, among others, the CC bonds of the hydrocarbons, and many other bonds that are recognizable as single. There are two other plateaus: one plateau around 1.4 contains many aromatic bonds , and a small plateau at 1.8, which contains double bonds. There are also some points in the extreme that correspond to triple bonds, but these are underrepresented in our study .
What Is The Importance Of Bond Order
It is important to study the bond order formula in chemistry. It helps us to understand several factors contributing to the formation of the compound. Some of them are:
Bond order helps us to know the number of participating electrons in the formation of bonds.
-
Bond order helps us to understand the stability of the bond. Higher bond order confers more stability.
-
Bond order helps us to understand the bond length.
-
Bond order helps us to understand bond strength. Higher bond order implies more energy is required to break the bond.
-
Bond order gives us an indication of the hybridization of the molecule.
-
A fractional bond order value implies that no bond is formed.
Read Also: Unit 1 Test Study Guide Geometry Basics Gina Wilson
A Discussed Organometallic Complex
and the FeC bond account for a somewhat total delocalization index of 1.7, which seems quite close to a 4 double bond, as would be expected from simple electron-counting techniques. Although the value of the DI in coupled cluster theory is significantly larger , this is a result of the increased delocalization in the TMM.
Bond Energy Or Bond Enthalpy
Bond energy is a measure of the strength of a chemical bond. It can be defined as the energy required to break all covalent bonds of a specific type in one mole of a chemical compound .
It is important to note that bond energy is not the same as bond dissociation energy. The latter is the change in enthalpy associated with the homolytic cleavage of a bond whereas the former is the average of the bond dissociation enthalpies of all bonds in a molecule.
You May Like: Algebra Nation Section 8 Answers
What Is The Difference Between Multiplicity And Bond Order
The concept of multiplicity is important in quantum chemistry, while the concept of bond order is important in molecular dynamics. The key difference between multiplicity and bond order is that multiplicity refers to the number of possible orientations of the spin of energy level, whereas bond order refers to a measurement of the number of electrons in chemical bonds.
The equation for the determination of multiplicity is 2S+1 where S is the total spin angular momentum. The equation for the determination of bond order is /2. Moreover, the multiplicity is measured as a relative value . But, the bond order is a particular value for a particular chemical bond. Usually, if the bond order is zero, it means there is no chemical bond.
Below infographic summarizes the difference between multiplicity and bond order.
Dihydrogen With An Electron In The Antibonding Orbital
In the second diagram, one of the bonding electrons in H2 is promoted by adding energy and placing it in the antibonding level.
Bond \ Order = \frac = 0
The above formula verifies breaking the H2 bond, which in this case gives a bond order of zero. For a bond to be stable, the bond order must be a positive value.
Dihydrogen with an electron in the antibonding orbital
Recommended Reading: Geometry Wars 3 Achievements
Tips For Calculating Bond Order:
In order to determine the bond order between two covalently bonded atoms by molecular orbital theory:
Bond Order = / 2
What Is Bond Order
The bond’s order determines the stability of a molecule or ion. The higher the bond order, the stronger is the bond, thus the higher the bond energy. Also, for diatomic molecules, a greater bond order means shorter bond length.
There are a number of bond order definitions. According to valence bond theory, it is
the number of bonded electron pairs between two atoms.
In molecular orbital theory, bond order is
the difference between the number of bonding electrons and the number of antibonding electrons divided by 2.
What’s the difference? Both of these theories refer to the position of electrons. While the first one is rather simple, the second one comes from quantum mechanics. We will try to explain it as simply as possible.
Our calculator uses the bond order formula from molecular orbital theory. In the next paragraph, we will explain how to use it. At the end of the text, you can also read about how to find bond order using Lewis structures.
Recommended Reading: How To Convert In Chemistry
How To Find Bond Order
Let’s remind ourselves what is bond order once again. The bond order is equal to the number of chemical bonds between two atoms. If between two atoms there is a single bond – the bond order is one. If there’s a double bond, bond order is two, and so on. To determine bond order, all you have to do is draw the Lewis structure of a molecule and establish what type of bond is between the atoms. Let’s look at some examples:
Bond order = number of bonds = 2
Bond order = 3
If you want to find bond order for a non diatomic molecules, you can do that by finding the average of bonds between pairs of atoms. For example:
Bond order = number of bonds / number of groups = 4 / 3 = 1.33
While bond order calculations are easy, finding bonding and antibonding electrons might get tricky! Come back to our bond order calculator whenever you need a quick reminder on how to calculate bond order. And before you go, check out this cell EMF calculator!
The Subtler Bonding Effects
In terms of the first response, the DI is an indicator of covalency: it measures the amount of electron pairs shared between two atoms. Thus, apolar bonds will return larger DI values than polar bonds because then no atom has a greater electronegativity than another atom, and thus the electrons are equally shared. On the other hand, in polar bonds, one of the atoms tends to hoard the electrons, making equal sharing less favourable. A clear example of this effect is that of N2 and HCN where = 3.043 and = 2.340, at HF level. This phenomenon can also be read in terms of the partition of electrons into localized and delocalized sets. For instance, on changing from a two-center, two-electron non-polar link to a polar one, the electrons that were originally delocalized between the two centers tend to localize toward the electronegative atom. From eqn it follows that the increase in the number of localized electrons must necessarily be accompanied by a decrease in the number of delocalized electrons. The end results is that polarity decreases the DI, as we observed for LiH and NaCl vide supra.
In terms of the second response, the DI recovers all textbook bonding effects. Here we will focus on two effects that are ubiquitous throughout chemistry: the inductive effect and the resonance effect. We discuss these two effects in turn, starting with the inductive effect.
Don’t Miss: How Many Physics Questions Are On The Mcat
Atomic Resolution Of Identity
As already noted, the concept of atoms within a molecule is not a well cut one. Strictly speaking, the atoms do not directly appear in the quantum mechanical description of molecules: the Schrödinger equation is written down for the individual particles . Thus one has to introduce some definition of the atom âfrom outside,â which necessarily means some arbitrariness. The Hilbert space analysis considered earlier is based on the fact that practical quantum chemistry mostly uses atom-centered basis sets. However, neither the use of basis atom-centered basis sets nor the Hilbert-space approach to the analysis is obligatory. A widely used alternative is the 3D analysis in which the physical space is decomposed into regions attributed to the individual atoms.
The âFuzzy Atomsâ Case
Our program FUZZYâit may be downloaded freely and is applicable after a âGaussianâ runâperforms calculations of both Hilbert space and fuzzy atoms bond orders and valences and fuzzy atoms overlap populations, for the case of both SCF and correlated wave functions. Programs BORDER and BO-VIR, applicable also for large systems, are presently designed for the SCF case only, but would require only minor changesâinput of the density matrices and not orbital coefficientsâfor the correlated calculations.
What Is The Bond Order Of No And No+
Answer:
The bond order shows the number of chemical bonds present between a pair of atoms. The Bond Order Formula can be defined as half of the difference between the number of electrons in bonding orbitals and antibonding orbitals.
Bond order formula is given as below\
a = Number of electrons in bonding molecular orbitals.
b = Number of electrons in antibonding molecular orbitals.
Read Also: Where Is Beth Thomas Now
The Mixed Second Quantization Formalism
In practice we use atom-centered basis sets, and then the quantum mechanical problem of describing the molecular electronic structure is solely defined by the one- and two-electron integrals over the basis AOs. The overlap of the basis orbitals centered on different atoms reflects chemically very important interactions, but makes difficult the calculation of the matrix elements of different operators. The mixed second quantization formalism permits to work in a relatively simple way with wave functions built up of nonorthogonal orbitals, by using essentially the same techniques that one applies in the case of an orthonormalized basis set, with the expense that the annihilation operators are defined in a special manner, and do not coincide with the adjoints of the respective creation operators.
PPDPP
After publication of my paper, I got a letter from Ms. Giambiagi with a copy of their paper in which essentially the same definition was proposed for the âall valence electronâ semiempirical theories with overlap as a formal generalization of the Wiberg index. Their paperâprobably because of the use of unusual and rather cumbersome notations and too lapidary a presentationâdid not receive the proper attention in the literature.
PsPP