Periodic Table Block Names
The block names come from the electron azimuthal quantum number values, which represent characteristics of spectroscopic lines: sharp , principal , diffuse , or fundamental . G-block gets its name because g is the next letter after f.
The four periodic table blocks are the basis for the main group , transition metal , and inner transition metal elements.
What Is The Change In An Ice Table
If the described change is unspecified, then the equilibrium concentration is listed in terms of x, and the equilibrium constant is used to solve for the variable. If the described change is specific, then the equilibrium concentration is listed as a concrete number, and it is used to solve for the equilibrium constant. We will explore both examples below.
Le Chateliers principle is especially relevant to ICE tables because the change in the ICE table represents the shift in the position of equilibrium: that means that if the change shows an increase in concentration of reactants, then there will be a subsequent decrease in the concentration of products. Vice versa as well: if the change shows a decrease in concentration of reactants, then there will be a subsequent increase in the concentration of products. This subsequent increase/decrease shows the position of equilibrium shifting to counteract the change to the reactants.
Additionally, Le Chateliers principle is relevant because the coefficient of the reactant or product affects the change to its concentration. For example, if a reactant has a coefficient of 1, then it changes by +x, but if a reactant has a coefficient of 2 or more, then it changes by +2x.
What Are F Block Elements
Elements whose f orbital getting filled up by electrons are called f block elements. These elements have electrons, in the f orbital, in the d orbital of the penultimate energy level and in the outermosts orbital.
There are two series in the f block corresponding to the filling up of 4f and 5f orbitals. The elements are 4f series of Ce to Lu and 5f series of Th to Lw. There are 14 elements filling up the f orbital in each series.
The position of F Block Elements in the Periodic Table: F block elements are placed separately at the bottom of the periodic table. They are a subset of 6th and 7th periods.
Recommended Reading: Analytic Geometry Unit 1 Similarity Congruence And Proofs
What Is Block Scheduling And Is It Effective Pros And Cons
Not all schools adhere to the same scheduling systems. Between the wide array of online schools to traditional schools, there are different possibilities within education for how to manage students and teachers time. If youve ever heard of the term block schedule and wondered, What is block scheduling? we are about to shed light on everything you need to know about this scheduling method.
Photo by javier trueba on Unsplash
Some Important Compounds Of P

Ammonia: Ammonia is a nitrogen and hydrogen compound that is necessary for life. It is formed as a result of the regular decay of vegetable and animal bodies. The demise and rot of animals and plants cause the nitrogen compounds found in them to deteriorate, resulting in the production of ammonia. Ammonia, in the form of ammonium salts, is present in the soil.
Ammonia is a type of gas. It is devoid of colour. It has a strong, pungent odour and a soapy taste. When it is suddenly inhaled, it attacks the eyes, causing tears. It is less dense than air. It dissolves easily in water. At room temperature and a pressure of around 8-10 atmospheres, ammonia easily melts.
Ozone: Ozone is an oxygen allotrope. It has a high level of instability in nature. Its traces can be found about 20 kilometres above sea level. It is created when oxygen reacts with the suns ultraviolet rays. This gass primary function is to shield the earths surface from harmful ultraviolet radiation from the sun. We use chlorofluorocarbons in refrigerators and other aerosols, which emit harmful substances into the air, causing gaps in the layer. As a result, we get UV light, which causes a lot of skin problems and cancer in people.
Don’t Miss: What Was The Geography Of The Southern Colonies
Reactions Of Manganese Compounds
i) Mn2+ with sodium hydroxide
Pale pink aqueous manganese ions react with sodium hydroxide to form a pale pinky-brown precipitate of manganese hydroxide, which darkens on standing in air. The precipitate is does not redissolve or react further on further addition of sodium hydroxide.
Mn2+ + 2OH Mn2
ii) Mn2+ with ammonia solution
It is only the hydroxide ions in the ammonia solution that react with manganese ions to form manganese hydroxide exactly as above. There is no further reaction with the ammonia present, and the precipitates do not redissolve.
Physical Properties Of S
- In the S block elements, the density of the alkali metals increases down the group. Exception: the density of potassium is less than the density of sodium.
- The alkali metals have a low melting and boiling point due to the weak metallic bonding.
- Alkali metals and their respective salts have the capability to impart colour to the oxidizing flame due to the heat generated from the flame which excites the valence electrons from one energy level to another energy level. This helps in the detection of alkali metals during the flame test.
Also Check: Math Magic Tricks With Algebra
Biological Role Of Ligand Substitution
Haemaglobin in blood is responsible for the transport of oxygen to cells for respiration, and the transport away of carbon dioxide.
Haemaglobin is a complex protein, containing four non-protein components called haem groups, each of which has an Fe2+ ion at its centre, and four dative covalent bonds between the iron and four N-atoms in the haem structure, and a further dative covalent bond to the protein globin.
Oxygen can bind to the iron in the haem group as a ligand, giving a co-ordination number of 6, in order to be transported. The colour of the complex when oxygen is bound as a ligand is the rich red of oxygenated blood.
Carbon monoxide can also bind to the iron at the same binding site as the oxygen would, the stability constant for this complex is greater so the bond between the iron and the CO is stronger and much less likely to break. CO will therefore bind preferentially if both carbon monoxide and oxygen are present in the lungs the binding is irreversible, so that haemaglobin becomes useless. Since tobacco smoking produces carbon monoxide, and this is one of the reasons why smokers become short of breath.
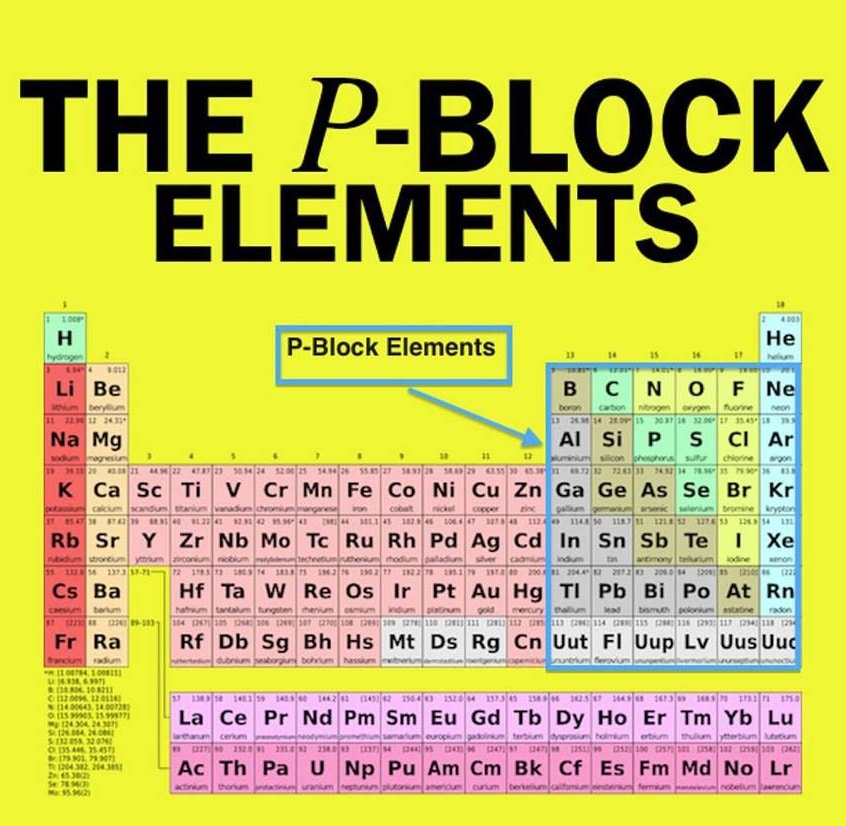
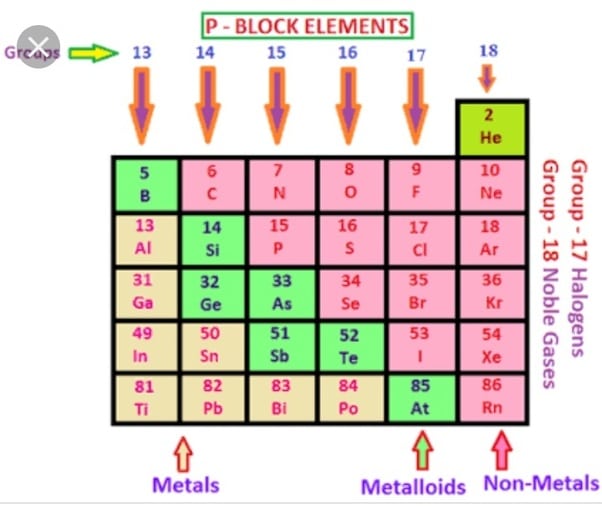
What Is The General Electronic Configuration Of P Block Elements
The general electronic external configuration for p block components is ns2np. dns is the general electronic outer configuration of d block components. The general electronic outer F block element configuration is fdns2.
To know more about p-block elements, register with BYJUS and download our app
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
Recommended Reading: What Does Equivalent Mean In Math
Reactions Of Iron Compounds
i) Fe2+ and Fe3+ with sodium hydroxide
Pale green iron ions in solution react with sodium hydroxide to form a dirty green precipitate of iron hydroxide. On standing the precipitate turns brown at its surface as iron hydroxide is oxidized to iron hydroxide.
Fe2+ + 2OH Fe2
Pale yellow iron ions in solution react with sodium hydroxide to form an orange-brown precipitate of iron hydroxide.
Fe3+ + 3OH Fe3
Neither precipitate redissolves on addition of excess sodium hydroxide.
ii) Fe2+ and Fe3+ with ammonia solution
It is only the hydroxide ions in the ammonia solution that react with iron and iron ions to form iron hydroxide and iron hydroxide exactly as above. There is no further reaction with the ammonia present, and the precipitates do not redissolve.
iii) oxidation of iron with acidified managanate
Pale green aqueous iron ions will decolourise purple managante ions in acidic solution in a redox reaction. The iron is oxidized to pale yellow aqueous iron ions, while the manganate is reduced to pale pink aqueous manganese ions.
MnO4 + 8H+ + 5Fe2+ 5Fe3+ + Mn2+ + 4H2O
iv) reduction of iron with iodide ions
Pale yellow aqueous iron ions can be reduced to pale green iron ions by redox reaction with colourless aqueous iodide ions. In this reaction iodine is formed in aqueous solution, which is brown, and masks the colour change of the iron ions.
2Fe3+ + 2I I2 + 2Fe2+
What Does Research Say
While its hard to say one way is better than another, some studies have found that block scheduling is ineffective. Some research has shown that students in the block schedule format have scored lower in science, biology, physics, and chemistry.
Block schedules may be harder on students because of the pace and lack of continuity. For teachers, it means developing longer lesson plans that should be compressed in a shorter amount of days.
Photo by ThisisEngineering RAEng on Unsplash
Don’t Miss: What Is K+ In Chemistry
Periodic Table Blocks Of Elements
Periodic table blocks are sets of elements grouped by their valence electron orbitals. The four block names are s-block, p-block, d-block, and f-block. Should a new element be discovered, it will be in g-block. Each block indicates which electron sublevel is in the process of being filled.
Charles Janet introduced the concept of element blocks as an alternative to element groups .
Relationship Between S P D And F Blocks And Electronic Configuration
The labels s, p, d and f blocks of the Periodic Table refer to the subshell that is being filled with electrons.
Group 1 elements occur at the beginning of a new row of the Periodic Table. The highest energy level contains only 1 electron in an s subshell.
Group 2 elements occur directly to the right of Group 1 elements.The highest energy level contains 2 electrons, both electrons occupy an s subshell.The s subshell for this energy level is now full.
The highest energy level of a Group 13 element already has 2 electrons in an s subshell, so the next electron occupies a p subshell to make 3 valence electrons in total . As you proceed from left to right across the Period from Group 13 to Group 18 elements, electrons are being added to the p subshell. Group 18 elements have 2 s electrons and 6 p electrons in their highest energy level which completes the s and p subshell.
Transition metals are filling their d subshell with electrons, starting with Group 3 elements which have 1 electron in a d subshell. Group 12 elements have 10 electrons in a d subshell, which corresponds to a completed d subshell.
Lanthanoids and actinoids are filling their f subshells with electrons.
Recommended Reading: How Does Quantum Physics Explain Spirituality
What Are The Properties Of Non
Usually non-metal is brittle when it is solid and typically has low thermal conductivity and electrical conductivity. Chemically, non-metals tend to have relatively high energy from ionization, contact with electrons, and electronegativity. As they react with other elements and chemical compounds, they receive or exchange the electrons.
Diagonal Relationship Within S
A diagonal relationship in S block elements exists between adjacent elements which are located in the second and third period of the periodic table. For example, Lithium of group 1A and second period shows similarities with the properties of magnesium which are located in the 2nd group and 3rd period.
Similarly, properties of beryllium which are located in the 2nd group and 2nd period show a likeness with properties of aluminium which is located in the third period and third group. The two elements which show similarities in their properties can be called a diagonal pair or diagonal neighbours.
The properties of S block elements vary significantly when compared to the other elements of the sub-group they belong to. The diagonal neighbours show a lot of similarities. Such a relationship is exhibited as you move left to right and down the group the periodic table has opposing factors.
For example, the electronegativity of the S block elements increases as we go across the period and decreases as we go down the group. Therefore, when it is moved diagonally the opposite tendencies cancel out and the value of electronegativity almost remains the same.
Recommended Reading: What Happened To Cool Math Games
Isomerism In Complex Ions
Stereoisomerism arises when the ligands can be arranged in different spatial arrangements. In complexes, as with organic substances, we will see both cis-trans isomerism and optical isomerism.
Cis Trans isomerism
When a complex contains four of one ligand and two of another, we can have cis and trans isomers.
e.g. cobalt forms an octahedral complex of formula +. The cobalt is in oxidation state +3 here. When salts containing this complex ion were crystallised, it was found that a green salt and a purple salt both with the same formula could be crystallized. This was clear evidence for two different isomers:
Cis-trans isomerism is also possible in some complexes with co-ordination number 4 if they have a square-planar configuration.
Nickel forms a square planar complex . This has no overall charge, as the nickel is in the +2 oxidation state.
A similar complex of platinum is one of the most effective drugs in chemotherapy for cancer. It is the cis-form of the complex which is biologically active. The drug is a liquid, usually administered intravenously as a drip, and goes under the name of cis-platin.
The importance of the exact shape and structure of the molecule is emphasized by the fact that the trans-form is ineffective.
Optical isomerism
Examples:
2+ a complex with three bidentate ligands
S Block Elements For Iit Jee
The elements of Group 1 and Group 2 of the modern periodic table are called S-block elements. Two types of s block elements are possible i.e. the elements with one electron or the elements with two electrons in their s-subshell.
S-block comprises 14 elements namely hydrogen , lithium , helium , sodium , beryllium , potassium , magnesium , rubidium , calcium , cesium , strontium , francium , barium , and radium .
Read Also: What Kind Of Physics Is On The Mcat
Post Reactions Of Block Copolymers
Block copolymers of structures not attainable directly by polymerization reactions can sometimes be prepared by the chemical modification of more readily accessible precursor block polymers. No general principles can be laid down, but examples include: the quaternization of amine group-containing blocks to give polyionic products the hydrogenation of double bonds in block copolymers derived from dienes, yielding saturated sequences and the removal of protecting groups in block copolymers to reveal sensitive groups that could not have survived during the construction of the main chain.
Anthony J. Ryan, … Ian W. Hamley, in, 2000
Health Effects Of Dietary Carbohydrate Restriction
Low-carbohydrate diets may miss the health advantages such as increased intake of dietary fiber afforded by high-quality carbohydrates found in legumes and pulses, whole grains, fruits, and vegetables. A “meta-analysis, of moderate quality,” included as adverse effects of the diet halitosis, headache and constipation.
Carbohydrate-restricted diets can be as effective as low-fat diets in helping achieve weight loss over the short term when overall calorie intake is reduced. An Endocrine Society scientific statement said that “when calorie intake is held constant body-fat accumulation does not appear to be affected by even very pronounced changes in the amount of fat vs carbohydrate in the diet.” In the long term, effective weight loss or maintenance depends on calorie restriction, not the ratio of macronutrients in a diet. The reasoning of diet advocates that carbohydrates cause undue fat accumulation by increasing blood insulin levels, and that low-carbohydrate diets have a “metabolic advantage”, is not supported by clinical evidence. Further, it is not clear how low-carbohydrate dieting affects cardiovascular health, although two reviews showed that carbohydrate restriction may improve lipid markers of cardiovascular disease risk.
Recommended Reading: Compact Numerical Methods For Computers Linear Algebra And Function Minimisation
Similarities Between Lanthanides And Actinides
The elements of lanthanide and actinide series are characterized by filling of f subshell. They possess almost similar outermost electronic configuration hence have similar properties. Following are the significant similarities:
Performance Characteristics Of Obcs
OBCs made by chain shuttling between catalysts incorporating large and small amounts of comonomer, respectively, can produce polymer microstructures which block the comonomer, thus exhibiting crystallization behavior that is distinct from that of RCPs of equivalent crystallinity. These novel microstructures extend the traditional regime of flexibility and heat resistance for olefin-based TPEs. Figure 31 compares the dynamic storage modulus versus temperature for two random olefin elastomers and an OBC with similar crystallinity.
Figure 31. Plot of storage modulus vs. temperature for statistically random ethyleneoctene and propyleneethylene copolymers compared to an ethyleneoctene OBC.
et alMetal Catalysts in Olefin Polymerization. Topics in Organometallic Chemistry
Compared to the ethyleneoctene RCP and propyleneethylene RCP, the OBC shows a plateau modulus that extends beyond 100 °C until the melting point of the high crystalline hard blocks is reached. The moduli of the RCPs decrease quickly after 50 °C, which signifies that the softening or melting point has been reached. Based on the architecture of the elastomer, the OBC also exhibits a low Tg that is characteristic of ethyleneLAO elastomers containing high comonomer levels. This is contrasted with the propyleneethylene RCP, which exhibits a higher Tg that arises from its propylene crystallinity.
Figure 32. Engineering stressstrain curves comparing an OBC and a random copolymer.
et al
G. Liu, in, 2011
Don’t Miss: How To Master Human Psychology