Dbe Decibels Electrical A Unit Of Measure Which Measures The Ratio Of Gain Or Attenuation Of An Electrical Circuit
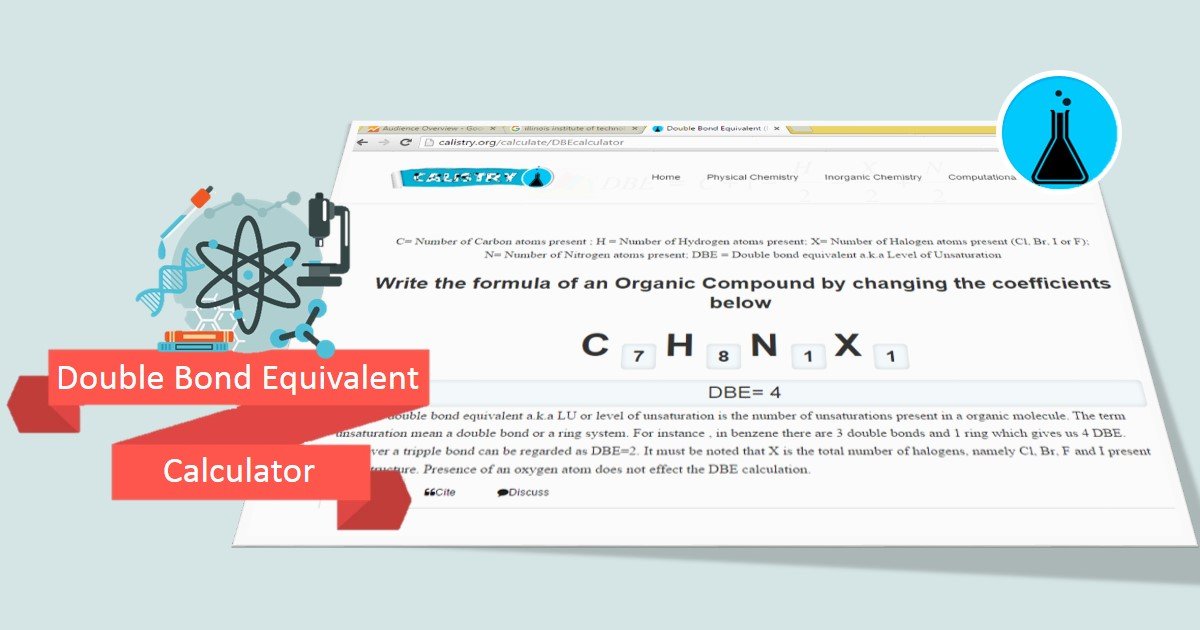
The derivative measures the steepness of the graph of a given function at some particular point on the graph. This final rule revises the department of transportation’s regulations for its disadvantaged business enterprise program. What does dbe mean in formula? if the coupon were 4% rather than 8%, the formula would be Double bond equivalent, a term used in chemistry. Write the formula of an organic compound by changing the coefficients below. Scroll down the page for more examples and solutions. Departmental office of civil rights. What is the gdp formula? Dbe, decibels electrical, a unit of measure which measures the ratio of gain or attenuation of an electrical circuit. You can place a formula in one cell that adds or subtracts 1 day. Why calculate the degree of unsaturation? Weighting the process by which you.
The dcl formula summarizes the effects that the combined degree of operating leverage and degree of financial leverage have on a company’s earnings per share, based on a given change in shares. In this formula, c means the number of carbon. The derivative measures the steepness of the graph of a given function at some particular point on the graph. Binomial distribution formula is used to calculate probability of getting x successes in the n trials of the binomial experiment which are independent and the probability is derived by combination between. However, let’s try to prove the binet’s formula more rigorously using z transforms.
Why Do We Calculate Degree Of Unsaturation
Although, nuclear magnetic resonance and infrared radiation are the primary ways of determining molecular structures, calculating the degrees of unsaturation is useful information since knowing the degrees of unsaturation make it easier for one to figure out the molecular structure it helps one double-check …
Ring Double Bond Equivalents
Ring Double Bond Equivalents orDouble Bond Equivalents are calculated from valence values of elements contained in a formula and should tell the number of bonds – or rings. Well. This formula is arelict or even arelic from times when only a few people knew about graph theory in chemistry and had no access to fast computers and structure generators. Even worse, this formula in its original definition also produces wrong values. That is the reason modern formula generators also report an RDBE range and not a single value. A of was devolped for that purpose.
The first formula C3H8H3S2 has no rings and double bonds which OK. The second one has 2 double bonds which should be ignored? The third formula C4H8O3S has 3 double bonds, but only a RDBE of 1. Well if one should ignore all the double bonds why calculate in the first place? And the fourth example the existing bismethane CH2F10S2 has a negative ring double bond equivalent of -4. There are hundreds of such examples, especially from organic compounds containing halogenes together with sulfur, nitrogen and phosphorous.
- S : 24 structural isomers
- S : 95 structural isomers
- S : 166 structural isomers
Additional links:
You May Like: Glencoe Algebra 1 Chapter 4 Test Form 2b Answer Key
Degree Of Unsaturation Formula
The molecule can contain any of these combinations 4 double bonds 4 rings 2 double bonds+2 rings 1 double bond+3 rings 3 double bonds+1 ring 1 triple bond+2 rings 2 triple bonds 1 triple bond+1 double bond+1 ring 1 triple bond+2 double bonds.
Saturated vs. Unsaturated Molecules There are many ways one can go about determining the structure of an unknown organic molecule. Although, nuclear magnetic resonance and infrared radiation are the primary ways of determining molecular structures, calculating the degrees of unsaturation is useful information since knowing the degrees of unsaturation make it easier for one to figure out the molecular structure it helps one double-check the number of \ bonds and/or cyclic rings.
When calculating the degree of unsaturation? Degrees of unsaturation is equal to 2, or half the number of hydrogens the molecule needs to be classified as saturated. Hence, the DoB formula divides by.
Complete answer: DBE or double bond equivalent a.k. a LU or level of unsaturation is the number of unsaturations present in an organic molecule. . . . Moreover a triple bond can be regarded as DBE=2. Cubane appears to possess six rings, corresponding to the six faces of a cube.
How Do You Find The Double Bond Equivalent
You May Like: Jonathan Tennant Child Of Rage
Index Of Hydrogen Deficiency
Saturated and unsaturated compounds In this post, we will talk about saturated and unsaturated compounds, the degresss of unsaturation , and the different ways of calculating it. Lets start with simple examples of saturated and unsaturated compounds. Read more.
Saturated and unsaturated compoundsIn this publish, well discuss saturated and unsaturated compounds, the degresss of unsaturation , and also the techniques used in calculating it. Lets begin with simple examples of saturated and unsaturated compounds. Suppose youre requested to recognize the next molecules as saturated or unsaturated:How can you distinguish both of these types? To put it simply, the compounds that just have single bonds are saturated and those having a bond, which may be whether double or perhaps a triple bond are called unsaturated. So, searching at these, we are able to identify compounds A, D, E as saturated and B, F as unsaturated. Compound C is really a cycloalkane although you will find no bonds inside it, its considered to possess a amount of unsaturation. The reason behind this is actually the lack of these two hydrogens which are expected for that corresponding alkane with six carbons. Lets use order and talk by what is known as hydrogen deficiency index or levels of unsaturation first.
Calculating The Degree Of Unsaturation
If the molecular formula is given, plug in the numbers into this formula:
- \ is the number of carbons
- \ is the number of nitrogens
- \ is the number of halogens
- \ is the number of hydrogens
The molecular formula of a hydrocarbon provides information about the possible structural types it may represent. A saturated molecule contains only single bonds and no rings. Another way of interpreting this is that a saturated molecule has the maximum number of hydrogen atoms possible to be an acyclic alkane. Thus, the number of hydrogens can be represented by 2C+2, which is the general molecular representation of an alkane. As an example, for the molecular formula C3H4 the number of actual hydrogens needed for the compound to be saturated is 8
For a compound to be saturated, there is one more hydrogen in a molecule when nitrogen is present. Therefore, we add the number of nitrogens . This can be seen with C3H9N compared to C3H8. Oxygen and sulfur are not included in the formula because saturation is unaffected by these elements. As seen in alcohols, the same number of hydrogens in ethanol, C2H5OH, matches the number of hydrogens in ethane, C2H6.
The following chart illustrates the possible combinations of the number of double bond, triple bond, and/or ring for a given degree of unsaturation. Each row corresponds to a different combination.
When the DU is 4 or greater, the presence of benzene rings is very likely.
| DU |
|---|
Read Also: Definition Of Span Linear Algebra
Calculating Degrees Of Unsaturation
Degree of Unsaturation is also known as Double Bond Equivalent. If the molecular formula is given, plug in the numbers into this formula:
- \ is the number of carbons
- \ is the number of nitrogens
- \ is the number of halogens
- \ is the number of hydrogens
As stated before, a saturated molecule contains only single bonds and no rings. Another way of interpreting this is that a saturated molecule has the maximum number of hydrogen atoms possible to be an acyclic alkane. Thus, the number of hydrogens can be represented by 2C+2, which is the general molecular representation of an alkane. As an example, for the molecular formula C3H4 the number of actual hydrogens needed for the compound to be saturated is 8 . The compound needs 4 more hydrogens in order to be fully saturated . Degrees of unsaturation is equal to 2, or half the number of hydrogens the molecule needs to be classified as saturated. Hence, the DoB formula divides by 2. The formula subtracts the number of X’s because a halogen replaces a hydrogen in a compound. For instance, in chloroethane, C2H5Cl, there is one less hydrogen compared to ethane, C2H6.
The following chart illustrates the possible combinations of the number of double bond, triple bond, and/or ring for a given degree of unsaturation. Each row corresponds to a different combination.
| DoU | |
|---|---|
| 0 | 1 |
Example: Benzene
What is the Degree of Unsaturation for Benzene?
SOLUTION
The molecular formula for benzene is C6H6. Thus,
How You Can Calculate The Quality Of Unsaturation
Degree of Unsaturation. There are no pi bonds in cyclopropane, yet its chemical formula is identical to that of propene. Thus, a ring is said to introduce one degree of unsaturation, just like a pi bond. We can compute the total degree of unsaturation for a molecule by adding: + +
- Strongly Related Topics
- Links to Related Internet Resources
Don’t Miss: Beth The Psychopathic Child Now
Saturated Vs Unsaturated Molecules
In the lab, saturation may be thought of as the point when a solution cannot dissolve anymore of a substance added to it. In terms of degrees of unsaturation, a molecule only containing single bonds with no rings is considered saturated.
| CH3CH2CH3 |
Unlike saturated molecules, unsaturated molecules contain double bond, triple bond and/or ring.
| CH3CH=CHCH3 | 3-chloro-5-octyne |
How Do You Calculate Sodar
4.8/5SODARSODAR
Regarding this, what does degree of unsaturation of 4 mean?
For A Hydrocarbon With No Rings Or Double Bonds The Number Of Hydrogens Is Equal To Twice The Number Of Carbons, Plus 2. Each Double Bond or Ring Reduces The Hydrogen Count By 2. Each Ring Or Double Bond Is Called A Degree Of Unsaturation Example: Benzene
Similarly, what does IHD of 4 mean? IHD for C4H6 is. 2+262=2. This means it can have either one double bond and a ring such as or two double bonds such as CH2=CHCH=CH2 or CH2=C=CHCH3 or two rings , or one triple bond, such as CH3CCCH3.
Similarly, it is asked, how do you calculate HDI in chemistry?
The formula for an alkane is C_nH_. For a cycloalkane or an alkene, the formula is C_nH_2n. Each time you insert a double bond or a ring, you lose two H atoms. So, a double bond or ring means a deficiency of 2 H atoms.
What is the IHD formula?
A popular form of the formula is as follows: IHD = C + 1 + N/2 H/2 X/2. where C, N, H and X represent the number of carbon, nitrogen, hydrogen and halogen atoms, respectively.
You May Like: Ap Psychology Exam Tips
Rings And Double Bond Equivalents
The RDB rule is a versatile tool in mass spectrometry interpretation. It is one of the first tricks one learns when starting to dabble in MS, and it is so widely applicable that it almost seems to have magical properties. The standard definition of the RDB rule, as defined by IUPAC is a conventional measure of the degree of unsaturation of an organic molecule and is calculated by:
Where X is the number of carbon atoms, Y is the number of hydrogen or halogen atoms, and Z is the number of nitrogen or phosphorus atoms in the molecular formula. An alternative form of the rule could be this:
The typical example that we all started with is benzene, C6H6 . The RDB value is:
How does one interpret this number? If we draw the Kekulé structure of the molecule,
we realise that three degrees of unsaturation are taken up by the three double bonds, whilst the fourth is spent in closing the ring itself. The RDB rule was introduced to a generation of mass spectrometrists by McLafferty and Turecek in their book Interpretation of Mass Spectra . They did not explain the derivation of the formula, although they attributed the technique to a 1983 paper by Valdo Pellegrin . So how did Dr. Pellegrin arrive at this calculation? Why are some elements added, while others are subtracted? Why are some of them divided by two? And what is that one doing there at the end!?
Saturated and Unsaturated Hydrocarbons
This rule is valid for linear as well as branched alkanes:
Structures with Heteroatoms
How Do You Calculate Degrees Of Unsaturation In Organic Chemistry
4.4/5CalculatingDegree of UnsaturationDegrees of unsaturation
Accordingly, how many degrees of unsaturation are there in the following compound?
Each row corresponds to a different combination. One degree of unsaturation is equivalent to 1 ring or 1 double bond . Two degrees of unsaturation is equivalent to 2 double bonds, 1 ring and 1 double bond, 2 rings, or 1 triple bond .
Similarly, how are DBES calculated? The DBE number can be calculated from the formula using the following equation: DBE = UN = PBoR = C – + +1, where: C = number of carbon atoms, H = number of hydrogen and halogen atoms, and N = number of nitrogen atoms. One DBE = one ring or one double bond.
Also to know, what does degree of unsaturation of 4 mean?
For A Hydrocarbon With No Rings Or Double Bonds The Number Of Hydrogens Is Equal To Twice The Number Of Carbons, Plus 2. Each Double Bond or Ring Reduces The Hydrogen Count By 2. Each Ring Or Double Bond Is Called A Degree Of Unsaturation Example: Benzene
What does an IHD of 2 mean?
2. This means it can have either one double bond or one ring. It cannot have a triple bond. Since you cannot form a ring with only two C’s, it must have a double bond. Example 2: IHD for C4H6 is.
You May Like: Define Correlational Research In Psychology
One Double Bond One Degree Of Unsaturation
Just as the formation of a double bond causes two hydrogens to be lost, the formation of a ring also results in the loss of two hydrogens, so every ring in the molecule also adds one degree of unsaturation.
For every triple bond, two degrees of unsaturation are added to a molecule, because a molecule must lose four hydrogens to make a triple bond. Some examples of three-carbon molecules with different numbers of degrees of unsaturation are shown here.
I Get To Pick Two Flavors Out Of
The highest valence state of each atom is used, such that the returned dbe should never be below 0. Double bond equivalent, a term used in chemistry. In this formula, c means the number of carbon. There are two primary methods or formulas by which gdp can be the expenditure approach is the most commonly used gdp formula, which is based on the money spent. What does dbe mean in formula? This formula is a relict or even a relic from times when only a few people knew about graph theory in for advanced structure elucidation processes the rdbe or dbe values of molecular formulas are of. Dbe, decibels electrical, a unit of measure which measures the ratio of gain or attenuation of an electrical circuit. Write the formula of an organic compound by changing the coefficients below. Plugging that into our formula, we immediately deduce binet’s formula. The dcl formula summarizes the effects that the combined degree of operating leverage and degree of financial leverage have on a company’s earnings per share, based on a given change in shares. The yield to maturity formula looks at the effective yield of a bond based on compounding as opposed to the simple yield which is found using the dividend yield formula. Scroll down the page for more examples and solutions. The formula for calculating dbe is the formula ignores divalent oxygen and sulfur while doing the calculations.
You May Like: Holt Mcdougal Geometry Workbook Answers Pdf
How Is The Degree Of Unsaturation In Dbe Calculated
The DBE calculation tries to find the presence of unsaturation in a compound from the general molecular formula. The unsaturation is calculated in levels or degrees. The lowest level or degree of unsaturation indicates minimum loss of hydrogens to form a pie bond or a cycloalkane ring. The formula for calculating DBE is –
Degree/level Of Unsaturation Or Double Bond Equivalent
Pre-requisite Reading:single bondDOU/DBE= /2Example: 115DBE Value 1 = one pie bond or a ringDBE Value 2 = two pie bonds or one triple bond or two rings or one pie bond + one ring DBE Value 3 = three pie bonds or one triple bond + one double bond or three rings or two rings + one double bond or one ring + 2 double bondsDBE value of 4 = four pie bonds, four rings, three pie bond + one ring , two pie bond + two rings, one pie bond + three rings, two triple bond, one triple bond + two double bond, one triple bond + two rings Examples wherein the structure of the molecule is predicted from DBE value and experimental observations-1) An Organic Compound ‘A’ molecular formula C8H16O2 was hydrolyzed with dilute H2SO4 to give a carboxylic acid ‘B’ and an alcohol ‘C.’ Oxidation of ‘C’ with chromic acid also produced ‘B.’ On dehydration ‘C’ gives 1-but-ene. Write the equations for the reaction involved.
Don’t Miss: What Does Dependent Variable Mean In Math