Lab Simulations For Chemistry
Bring the world of science into the classroom or enable students to bring learning home with Labsters virtual science lab content. No need for additional hardware or lab equipment access these chemistry labs on any laptops, and spark creativity in students with this innovative and interactive way to explore science.
E: Reactions In Aqueous Solution
- Page ID
- 24803
These are homework exercises to accompany the Textmap created for “Chemistry: The Central Science” by Brown et al. Complementary General Chemistry question banks can be found for other Textmaps and can be accessed here. In addition to these publicly available questions, access to private problems bank for use in exams and homework is available to faculty only on an individual basis please contact for an account with access permission.
What Is An Aqueous Solution Example
Cola, saltwater, rain, acid solutions, base solutions, and salt solutions are examples of aqueous solutions. Similarly, if a mixture contains water but no solute dissolves in the water as a solvent, an aqueous solution is not formed. For example, mixing sand and water does not produce an aqueous solution.
Recommended Reading: Algebra Connections Volume 2 Answers
Aqueous Solution Facts For Kids
An aqueous solution is a solution where the solvent is water. In a chemical equation it is written by adding to the formula. A solution of ordinary table salt in water would be written as NaCl. NaCl is the part that means table salt. The word aqueous means being dissolved in water. Water is a solvent. It is found all over the place. Water is a solvent in chemistry.
Properties Of Aqueous Solutions
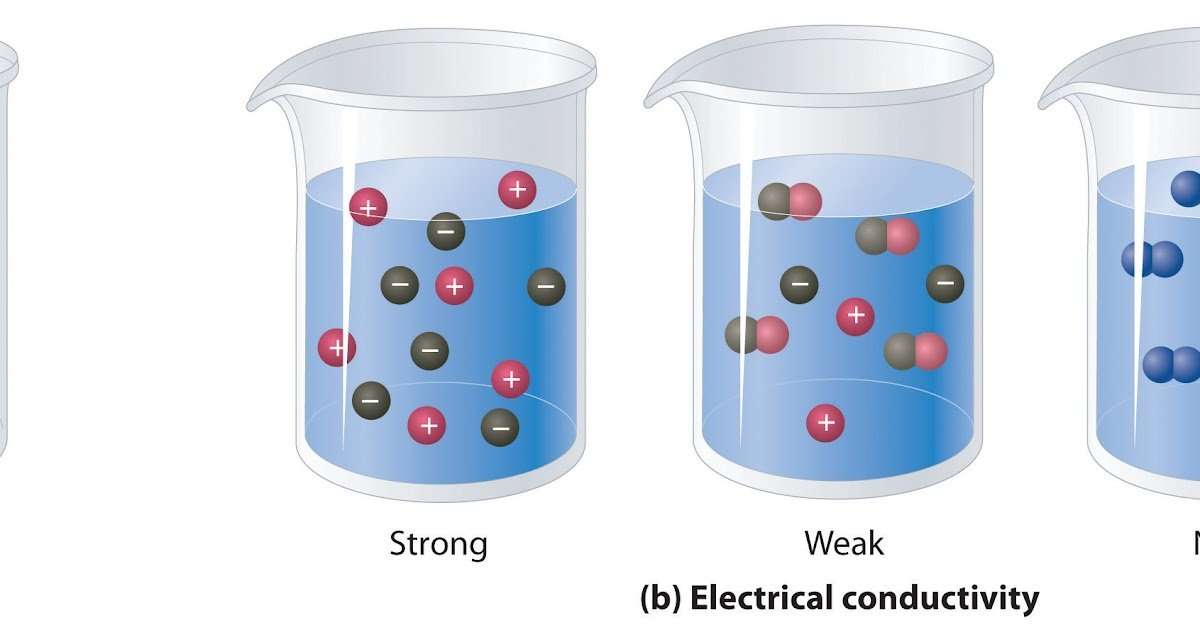
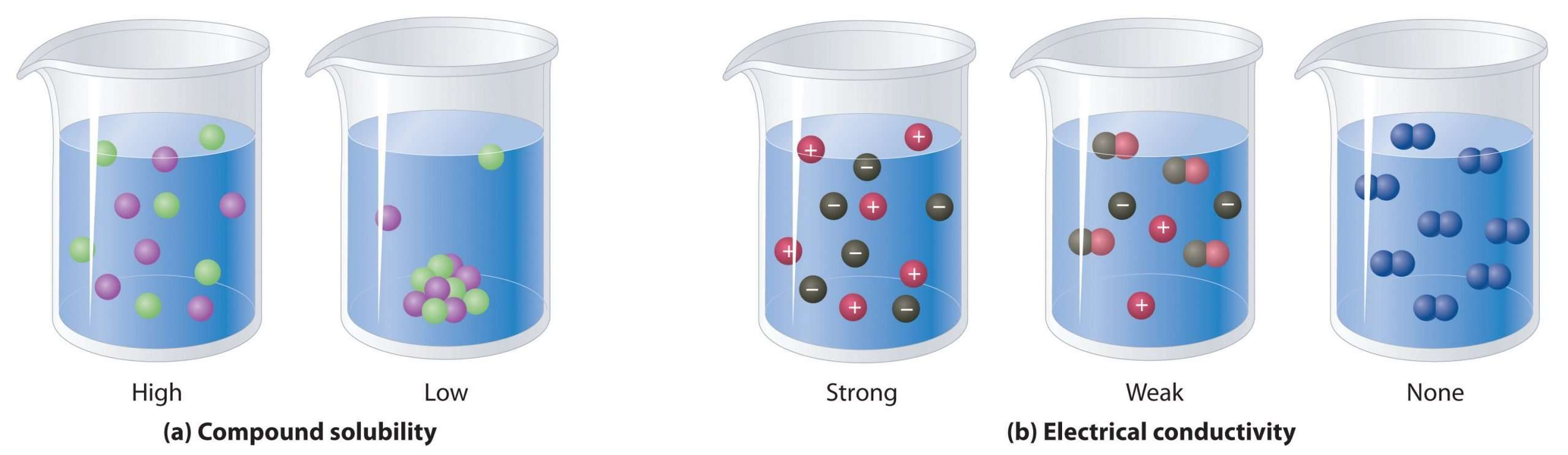
Aqueous solutions often conduct electricity. Solutions that contain strong electrolytes tend to be good electrical conductors , while solutions that contain weak electrolytes tend to be poor conductors . The reason is that strong electrolytes completely dissociate into ions in water, while weak electrolytes incompletely dissociate.
When chemical reactions occur between species in an aqueous solution, the reactions are usually double displacement reactions. In this type of reaction, the cation from one reactant takes the place for the cation in the other reactant, typically forming an ionic bond. Another way to think of it is that the reactant ions “switch partners”.
Reactions in aqueous solution may result in products that are soluble in water or they may produce a precipitate. A precipitate is a compound with a low solubility that often falls out of solution as a solid.
The terms acid, base, and pH only apply to aqueous solutions. For example, you can measure the pH of lemon juice or vinegar and they are weak acids, but you can’t obtain any meaningful information from testing vegetable oil with pH paper.
You May Like: Calculating Percent Error In Chemistry
What Is Aqueous Solution Of Aluminium Chloride
Aluminum Chloride figures among the most used Lewis acids and is one of the most powerful as well. Widely used as a catalyst, this compound is of major importance in organic chemistry. Examples of aluminum chloride use include alkylation of paraffin and aromatic hydrocarbons by olefins, the formation of complex ketones, aldehydes and carboxylic acid derivatives.
Aluminum chloride can be made by dissolving aluminum or aluminum hydroxide with hydrochloric acid. The solution, on concentration, deposits crystals of AIC13.6H20.
2AI + 6HCl 2AlCI3 + 3H2
In crystalline aluminum chloride, there are six water molecules as the water of crystallization. Hence its formula is AlCl3.6H2O.
Was this answer helpful?
Examples Of Aqueous Solutions
Both ionic and covalent solutes dissolve in water and form aqueous solutions. Examples of aqueous solutions include:
- Saline solution
- Vinegar
- Urine
Examples of non-aqueous solutions include any solutions in oil, hexane, benzene, toluene, or other solvents that are not water. When a substance combines with water and forms a mixture but does not dissolve, an aqueous solution is not formed. For example, mixing sand and water does not form an aqueous solution.
Also Check: Holt Geometry Chapter 7 Test Form A Answers
What An Aqueous Solution Really Contains
I want to know what an aqueous solution is. For example, let’s consider a $1~\mathrm$ aqueous solution of $\ce$. Does it also contain $\ce$? Though it is a simple question, I feel it is important to understand something as vital to chemistry as this.
- $\begingroup$It’s alright, everyone has trouble with this at first. 🙂 Aqueous means that the specific substance has been dissolved in $\ce$.$\endgroup$Apr 28, 2015 at 0:44
- $\begingroup$Then aqueous solution of MgCl2 means MgCl2 is dissolved in water$\endgroup$ On the way to successApr 28, 2015 at 0:46
- $\begingroup$Not really a simple question at all, but a topic that can cause difficulty. Good question!$\endgroup$ user15489Apr 28, 2015 at 2:40
- $\begingroup$water as solvent –> aqueous solution$\endgroup$ FreddyApr 28, 2015 at 16:15
- $\begingroup$If you are going to answer, please make sure that your answer adds something to discussion and does not rehash what others have already written.$\endgroup$
Uh… I think the insight you need is about chemical equilibrium.
An aqueous solution is exactly the mixture formed when something dissolved in water. This is easy to understand, but here is a more difficult question: what’s the component of this mixure?
To answer this is not easy.
In a glass of pure water, there are still a little $\ce $ and $\ce $. This is because sometimes proton will “jump” from one $\ce $ molecular to another one.
$\ce $.
$\ce $
$\ce $
For example, $\ce$ will meet an equilibrium:
$\ce$
Difference Between Liquid And Aqueous
Categorized under Science | Difference Between Liquid and Aqueous
Liquid vs Aqueous
A liquid is a state of matter. There being three states of matter, namely, solid, liquid, and gas. They all have their particular features and properties. By aqueous, we actually mean a solution where the solvent is water and some compound is dissolved in it.
LiquidsLiquidity is a state of matter. It has some typical characteristics which distinguish it from solids and gases. The first feature of liquid is that it can flow. Pour a glass of water at a slanting surface, and one can see it flowing from the higher to the lower surface. The second main feature is that it takes the shape of a container. When a liquid is poured into different shapes of containers, they take the shape of each container. When sealed in a container, they apply pressure evenly at all surfaces. The third most distinctive characteristic of liquids is the surface tension. The best example of surface tension is boiling milk in a container. Once it is boiled, it reaches the top and fluffs up like a fuzzy ball but does not immediately flow out. This characteristics leads to the phenomena called wetting.
Summary:
Also Check: Ct Algebra 1 Curriculum Version 3.0
What Is An Aqueous Solution Definition And Examples
An aqueous solution is a chemical solution in which the solvent is water. The solutes are dissolved molecules and ions that are surrounded by water molecules. An aqueous solution is shown by writing after a chemical formula. For example, an aqueous solution of salt in water is NaCl or Na+ + Cl-. In contrast, a solution in which the solvent is not water is called a non-aqueous solution.
What Is An Aqueous Solution In Chemistry
The aqueous solution containing water and at least one other item is indicated by the symbol after the substance. For example, salt water is a solution indicated by NaCl. Whereas, the components of salt in an aqueous solution are indicated by Na + Cl .
Water only dissolves hydrophilic items including acids, bases and salts. The aqueous solution of these items mixes together with the water completely. Hydrophobic items don’t dissolve very well in water, such as oils and fats.
When you dissolve electrolytes in water, the ions allow the solution to be conductive of electricity. Sugar is a nonelectrolyte and dissolves in water, but at the molecular level it stays intact so the solution is not conductive.
Also Check: Chapter 10 Test Form A Geometry Answer Key
Whats In Your Aqueous Cleaning Chemistry
HomeWhats in Your Aqueous Cleaning Chemistry?
Aqueous cleaning chemistries are an effective way to remove soils from parts. Organic soils, including motor oil and grease, and inorganic soils, such as scale and rust, can be eliminated with the right system and chemistry.
Every aqueous cleaners solvent is water. Unlike petroleum solvents, chemistries that are water-based clean without any harmful effects. Aside from water, whats in an aqueous parts washer solution?
Wetting agents, builders, sequestering agents, inhibitors and other materials are added to the chemistry, enhancing its cleaning ability. Whats added to the cleaning chemistry depends on the parts material and soils.
Cellulose As A Thermo
Aqueous solutions of most natural polymers form gels below a critical temperature usually known as the sol-to-gel transition temperature. Some cellulose derivates form a gel upon heating, with gelation temperatures of 4050 °C and 7590 °C for MC and HPMC, respectively. The transition temperature of HPMC can be lowered to ~40 °C by reducing the hydroxylpropyl molar substitution. The polymer chains are hydrated at lower temperatures while they start dehydrating as the temperature increases. Partial dehydration results in polymerpolymer association, thereby resulting in a network structure . Tate et al. developed MC-based scaffolds for the repair of brain defects. Their gels were biocompatible in both in vitro and in vivo conditions. Carlsson et al. reported a change in the thermal behavior of aqueous solutions of ethylcellulose with the addition of ionic surfactants such as sodium dodecyl sulfate or cetyl triammonium bromide. The systems underwent sol-to-gel phase transitions at 3040 °C, resulting in the formation of gels, with micelle-like surfactant clusters influencing gel properties. Scherlund et al. used this system for the sustained delivery of lidocaine and prilocaine in periodontal pockets.
Gerd Brunner, in, 2014
You May Like: Introduction To Exponential Functions Common Core Algebra 1 Homework
What Is Nonaqueous Solution
A nonaqueous solution is a solution we obtain by dissolving a solute in any solvent other than water. The solvent can be an organic compound such as acetone, toluene, ether, alcohol, benzene, etc.
Figure 02: Iodine in Alcohol
The solvent can be polar or nonpolar and depending on the polarity while solutes dissolve in the solvent. Solutions of iodine in alcohol and solutions of iodine in carbon tetrachloride are examples of nonaqueous solutions.
What Is The Difference Between A Liquid And An Aqueous Solution
A liquid has free flowing particles, meaning that is has a definite volume, but doesn’t have a definite shape. The most abundant liquid on Earth is water, as long as it’s at room temperature.
To be considered a liquid, all of the following properties have to be met:
They have to be almost incompressible. Their value only decreases slightly under pressure.
Liquid densities are affected by pressure but change very slightly when pressure is added.
Liquids always take the shape of any type of container they are in.
Liquids have surface tension causing wetting.
All the particles in a liquid have greater freedom to move about than in a solid state.
Related Articles
Read Also: How Did Geography Affect The Development Of The Greek City-states
Determining The Concentration Of An Unknown Solution Using A Titration
The chemical nature of the species present in the unknown dictates which type of reaction is most appropriate and also how to determine the equivalence point. The volume of titrant added, its concentration, and the coefficients from the balanced chemical equation for the reaction allow us to calculate the total number of moles of the unknown in the original solution. Because we have measured the volume of the solution that contains the unknown, we can calculate the molarity of the unknown substance. This procedure is summarized graphically here:
Difference Between Aqueous And Nonaqueous Solution
August 16, 2019 Posted by Madhu
The key difference between aqueous and nonaqueous solution is that thesolvent of an aqueous solution is water whereas, in nonaqueous solutions, the solvent is any substance other than water.
A solution contains a solvent and solute. The solutes are dissolved in the solvent. Here, the solutes and solvent should have the same polarity. Moreover, if the solvent is polar and solutes are nonpolar or vice versa, the solutes will not dissolve in the solvent, and we cannot obtain a solution.
Don’t Miss: Rationalizing Imaginary Denominators Worksheet Answers
Aqueous Solution Definition In Chemistry
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
An aqueous solution is any solution in which water is the solvent. In a chemical equation, the symbol follows a species name to indicate that it is in aqueous solution. For example, dissolving salt in water has the chemical reaction:
NaCl Na+ + Cl-
Although water is often called the universal solvent, it dissolves only substances that are hydrophilic in nature. Examples of hydrophilic molecules include acids, bases, and many salts. Substances that are hydrophobic do not dissolve well in water and tend not to form aqueous solutions. Examples include many organic molecules, including fats and oils.
When electrolytessuch as NaCl and KCldissolve in water, the ions allow the solution to conduct electricity. Nonelectrolytes like sugar also dissolve in water, but the molecule remains intact and the solution is not conductive.
Troubleshooting Your Cleaning Chemistry
Sometimes, parts cleaning doesnt go as expected. If parts arent coming out clean, you may need a higher concentration of the aqueous cleaner, increased pH levels or a higher temperature. Mechanics, time, heat and chemistry work in tandem to clean parts. If you run into trouble, try adjusting one of these cleaning factors or talk to your manufacturer for assistance.
Result: Part surfaces are dark after cleaning.
Possible Reason:
- The concentration of the aqueous chemistry is too high.
- The temperature of the solution is too hot.
- The cleaner chemistry is too aggressive for the material.
Result: Parts have a rainbow after cleaning.
Possible Reason:
- Temperatures are too high during cleaning and drying.
Result: The cleaning solution is yellow or brown.
Possible Reason:
- The temperature is too hot.
- The concentration is too high for the materials.
- Too much emulsified oil has collected in the solution.
- Air has oxidized the solution, causing it to darken.
Result: Part surfaces appear pitted after cleaning.
Possible Reason:
- The chemistry is too aggressive for the material. Switch to a lower pH or add more corrosion inhibitors.
How can you make sure an aqueous chemistry will work?
At Jenfab, we test wash your industrial parts for free before you make a purchase. Our test lab provides particle count and molecular weight analyses from before and after the test wash.
Are you ready to invest in an aqueous parts washer?
Also Check: Unit 1 Study Guide Geometry Basics Answer Key
Types Of Surfactant Cleaning Agents
- Anionic surfactants. Anionic surfactants have a negative charge and are most effective at lifting particulate soils. Examples of anionic surfactants are sulfates and gluconates.
- Nonionic surfactants. With a neutral charge, nonionic surfactants are excellent at emulsifying oils and cleaning metal. Nonionic surfactants are prone to separation depending on the temperature of the chemistry. When this happens, the cleaning solution becomes cloudy.
- Cationic surfactants. Lastly, cationic surfactants have a positive charge and can be used for antimicrobial purposes.
Example Aqueous Solution Chemistry Problems

Students encounter a few different types of chemistry problems concerning aqueous solutions. These mainly concern questions of solubility and colligative properties.
Example: Which solute forms an aqueous solution?
- sodium nitrate
- silver hydroxide
- copper sulfide
Technically, this is not a great question because allionic compounds form aqueous solutions, even if they are very poorly soluble. This is because ionic compounds, like water, are polar molecules. But, the point of a question like this is getting a student to understand solubility rules. Based on these rules, only sodium nitrate is highly soluble in water. Most carbonates, hydroxides, and sulfides are insoluble and these particular compounds are not exceptions to the rules.
Other common questions concern colligative properties. Colligative properties, like freezing point depression and boiling point elevation, depend on the number of particles dissolved in water. The more a compound dissociates into ions or the greater its concentration, the higher it raises boiling point or lowers freezing point.
Example: Which aqueous solution has the lowest freezing point?
- 0.1 molal urea solution
- 0.1 molal sucrose solution
- 0.1 molal sodium chloride solution
- 0.1 molal calcium chloride solution
Example: Which aqueous solution has the highest boiling point?
- 0.1 M NaCl
- 0.1 M CaCl2
- 0.1 M AlCl3
Read Also: What Is The Molecular Geometry Of Ccl4
Why Is An Aqueous Solution Important
In an aqueous solution where water is the solvent, the solute to be dissolved by the water has fewer particles in it, making the particles move in random motion. Pure water has a low concentration of ions and therefore does not conduct electricity. When a solute dissociates in water and forms an electrolyte, then the solution is a good conductor of electricity.
Solutes that dissociate in water and forms ions are electrolytes. Strong acids and bases in an aqueous solution form a strong electrolyte, which can dissolve completely as a soluble item. Weak electrolytes do not completely dissociate and are usually weak acids and bases. Since strong electrolytes supply ions to the solution, strong electrolytes create aqueous solutions that are more conductive of electricity.
Oxidation And Reduction At The Electrodes
Oxidation of ions or neutral molecules occurs at the anode, and the reduction of ions or neutral molecules occurs at the cathode. Two mnemonics for remembering that reduction happens at the cathode and oxidation at the anode are: Red Cat and An Ox . The mnemonic LeO said GeR is useful for remembering lose an electron in oxidation and gain an electron in reduction.
It is possible to oxidize ferrous ions to ferric ions at the anode. For example:
\text^\rightarrow\text^+e^
Neutral molecules can also react at either electrode. For example, p-Benzoquinone can be reduced to hydroquinone at the cathode:
+ 2 e^ + 2\text^ \rightarrow
Hydroquinone: Hydroquinone is a reductant or electron donor and organic molecule.
Para-benzoquinone: P-benzoquinone is an oxidant or electron acceptor.
In the last example, H+ ions also take part in the reaction, and are provided by an acid in the solution or by the solvent itself . Electrolysis reactions involving H+ ions are fairly common in acidic solutions, while reactions involving OH- are common in alkaline water solutions.
The oxidized or reduced substances can also be the solvent or electrodes. It is possible to have electrolysis involving gases.
In order to determine which species in solution will be oxidized and which will be reduced, the standard electrode potential of each species may be obtained from a table of standard reduction potentials, a small sampling of which is shown here:
Also Check: Glencoe Mcgraw Hill Geometry Workbook Answers