The Chemical Components Of A Cell
Matter is made of combinations of elementssubstances such as hydrogen or carbon that cannot be broken down or converted into other substances by chemical means. The smallest particle of an element that still retains its distinctive chemical properties is an atom. However, the characteristics of substances other than pure elementsincluding the materials from which living cells are madedepend on the way their atoms are linked together in groups to form molecules. In order to understand how living organisms are built from inanimate matter, therefore, it is crucial to know how all of the chemical bonds that hold atoms together in molecules are formed.
Evolution In Action: Carbon Dating
Figure 4. The age of remains that contain carbon and are less than about 50,000 years old, such as this pygmy mammoth, can be determined using carbon dating.
Carbon-14 is a naturally occurring radioisotope that is created in the atmosphere by cosmic rays. This is a continuous process, so more 14C is always being created. As a living organism develops, the relative level of 14C in its body is equal to the concentration of 14C in the atmosphere. When an organism dies, it is no longer ingesting 14C, so the ratio will decline. 14C decays to 14N by a process called beta decay it gives off energy in this slow process.
After approximately 5,730 years, only one-half of the starting concentration of 14C will have been converted to 14N. The time it takes for half of the original concentration of an isotope to decay to its more stable form is called its half-life. Because the half-life of 14C is long, it is used to age formerly living objects, such as fossils. Using the ratio of the 14C concentration found in an object to the amount of 14C detected in the atmosphere, the amount of the isotope that has not yet decayed can be determined. Based on this amount, the age of the fossil can be calculated to about 50,000 years . Isotopes with longer half-lives, such as potassium-40, are used to calculate the ages of older fossils. Through the use of carbon dating, scientists can reconstruct the ecology and biogeography of organisms living within the past 50,000 years.
Covalent Bonds Form By The Sharing Of Electrons
All the characteristics of a cell depend on the molecules it contains. A is defined as a cluster of atoms held together by here electrons are shared between atoms to complete the outer shells, rather than being transferred between them. In the simplest possible a molecule of hydrogen two H atoms, each with a single , share two electrons, which is the number required to fill the first shell. These shared electrons form a cloud of negative charge that is densest between the two positively charged nuclei and helps to hold them together, in opposition to the mutual repulsion between like charges that would otherwise force them apart. The attractive and repulsive forces are in balance when the nuclei are separated by a characteristic distance, called the bond length.
A further crucial property of any bondcovalent or noncovalentis its strength. Bond strength is measured by the amount of energy that must be supplied to break that bond. This is often expressed in units of kilocalories per , where a kilocalorie is the amount of energy needed to raise the temperature of one liter of water by one degree centigrade. Thus if 1 kilocalorie must be supplied to break 6 × 1023 bonds of a specific type , then the strength of that bond is 1 kcal/mole. An equivalent, widely used measure of energy is the , which is equal to 0.239 kilocalories.
Some energies important for cells. Note that these energies are compared on a logarithmic scale.
Recommended Reading: Segment And Angle Addition Worksheet Answers
The Chemistry Of Cells Is Dominated By Macromolecules With Remarkable Properties
On a weight basis, macromolecules are by far the most abundant of the carbon-containing molecules in a living cell . They are the principal building blocks from which a cell is constructed and also the components that confer the most distinctive properties of living things. The macromolecules in cells are polymers that are constructed simply by covalently linking small organic molecules into long chains . Yet they have many remarkable properties that could not have been predicted from their simple constituents.
Three families of macromolecules. Each is a polymer formed from small molecules linked together by covalent bonds.
What Are The Major Chemical Elements Found In Cells In Biology
The cells of living things are made mainly of four elements: carbon, hydrogen, oxygen and nitrogen. They make up 96% of the atoms that are in living things, so they would be considered major chemicals. However, depending on how you define major, other elements that only make up a few percent of cells can top the list. If major also means essential for life, then trace elements are very major though they make up just 0.5% of the atoms in an organism.
TL DR
The four most important elements in cells are carbon, hydrogen, oxygen and nitrogen. However, other elements — like sodium, potassium, calcium and phosphorus — are also important.
Recommended Reading: Equation To Find Half Life
Is Cast Iron Stronger Than Steel
Steel produces chips if it is grinded, and it is malleable. The strength of both cast iron and steel is also controversial, as some think steel is stronger than cast iron and others think that iron and steel are same thing, but the truth is that cast iron has a more compressive strength, and steel is more tensile.
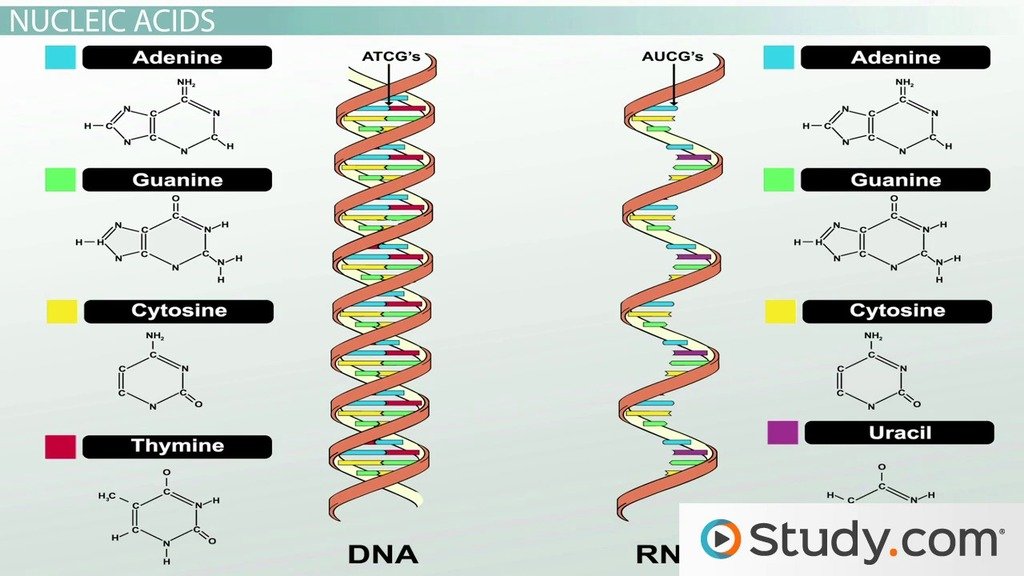
Sugars Provide An Energy Source For Cells And Are The Subunits Of Polysaccharides
The simplest the monosaccharidesare compounds with the general formula n, where n is usually 3, 4, 5, 6, 7, or 8. Sugars, and the molecules made from them, are also called carbohydrates because of this simple formula. Glucose, for example, has the formula C6H12O6 . The formula, however, does not fully define the : the same set of carbons, hydrogens, and oxygens can be joined together by covalent bonds in a variety of ways, creating structures with different shapes. As shown in , for example, can be converted into a different mannose or galactosesimply by switching the orientations of specific groups relative to the rest of the molecule. Each of these sugars, moreover, can exist in either of two forms, called the d-form and the l-form, which are mirror images of each other. Sets of molecules with the same chemical formula but different structures are called , and the subset of such molecules that are mirror-image pairs are called optical isomers. Isomers are widespread among organic molecules in general, and they play a major part in generating the enormous variety of sugars.
An Outline of Some of the Types of Sugars Commonly Found in Cells.
The reaction of two monosaccharides to form a disaccharide. This reaction belongs to a general category of reactions termed condensation reactions, in which two molecules join together as a result of the loss of a water molecule. The reverse reaction
You May Like: Hawkes Learning Systems Statistics Answer Key
The Top Four Elements Found In The Human Body
Of the elements found in the human body, four of them make up the largest percentage of our body weight . The four elements are oxygen, hydrogen, carbon, nitrogen. Before you start thinking we should float away with all the oxygen, hydrogen, and nitrogen atoms, remember that the oxygen molecules are mainly part of the water in our body . In fact, over half of the human body is made up of water .
The eleven common elements found in the human body and their percentage of total body weight. The other trace elements are: boron , cadmium , chromium , cobalt , copper , fluorine , iodine , iron , manganese , molybdenum , selenium , silicon , tin , vanadium , and zinc .
The Outermost Electrons Determine How Atoms Interact
To understand how atoms bond together to form the molecules that make up living organisms, we have to pay special attention to their electrons. Protons and neutrons are welded tightly to one another in the and change partners only under extreme conditionsduring radioactive decay, for example, or in the interior of the sun or of a nuclear reactor. In living tissues, it is only the electrons of an atom that undergo rearrangements. They form the exterior of an atom and specify the rules of chemistry by which atoms combine to form molecules.
The arrangement of an atom is most stable when all the electrons are in the most tightly bound states that are possible for themthat is, when they occupy the innermost shells. Therefore, with certain exceptions in the larger atoms, the electrons of an atom fill the orbitals in orderthe first shell before the second, the second before the third, and so on. An atom whose outermost shell is entirely filled with electrons is especially stable and therefore chemically unreactive. Examples are helium with 2 electrons, neon with 2 + 8, and argon with 2 + 8 + 8 these are all inert gases. Hydrogen, by contrast, with only one electron and therefore only a half-filled shell, is highly reactive. Likewise, the other atoms found in living tissues all have incomplete outer electron shells and are therefore able to donate, accept, or share electrons with each other to form both molecules and ions .
Also Check: Why Is Physics Considered To Be The Basic Science
Water Is The Most Abundant Substance In Cells
Water accounts for about 70% of a cell’s weight, and most intracellular reactions occur in an environment. Life on Earth began in the ocean, and the conditions in that primeval environment put a permanent stamp on the chemistry of living things. Life therefore hinges on the properties of water.
In each water the two H atoms are linked to the O atom by covalent bonds . The two bonds are highly because the O is strongly attractive for electrons, whereas the H is only weakly attractive. Consequently, there is an unequal distribution of electrons in a water molecule, with a preponderance of positive charge on the two H atoms and of negative charge on the O . When a positively charged region of one water molecule comes close to a negatively charged region of a second water molecule, the electrical attraction between them can result in a weak bond called a . These bonds are much weaker than covalent bonds and are easily broken by the random thermal motions due to the heat energy of the molecules, so each bond lasts only an exceedingly short time. But the combined effect of many weak bonds is far from trivial. Each water molecule can form hydrogen bonds through its two H atoms to two other water molecules, producing a network in which hydrogen bonds are being continually broken and formed . It is only because of the hydrogen bonds that link water molecules together that water is a liquid at room temperature, with a high boiling point and high surface tensionrather than a gas.
What Elements Are Found In The Human Body
There are 92 elements that occur naturally on Earth. For living things, only 11 of these elements are found in larger than trace quantities. Any amount 0.01% or less is considered a trace element. For vertebrates, such as humans, there are two additional elements that occur in larger than trace amounts these are Iodine and Iron. The periodic table of elements below is color coded to show the elements found in the human body.
Periodic Table illustration of elements found in human body. Click to enlarge.
Don’t Miss: Ccl4 Electron Pair Geometry
Examples Of Element In A Sentence
elementselementelement Milwaukee Journal Sentinelelement WSJelement chicagotribune.comelement The New Yorkerelement sun-sentinel.comelementForbeselement Washington Postelement Rolling Stone
These example sentences are selected automatically from various online news sources to reflect current usage of the word ‘element.’ Views expressed in the examples do not represent the opinion of Merriam-Webster or its editors. Send us feedback.
Water Is A Polar Molecule
Note also that the sharing of electrons is not always equal. For example, in a water molecule, the negatively charged electrons spend more time in the vicinity of the heavier oxygen atom.
The net result is that the water molecule has one end that is more negative relative to the other end. Water is therefore a “polar” molecule. We will see that this polarity has important implications for many biological phenomena including cell structure. You may have heard the expression “like dissolves like.” What this means is that polar molecules dissolve well in polar fluids like water. Sugars and salts are polar molecules, and they dissolve in water, because the positive and negative parts of the two types of molecules can distribute themselves comfortably among one another.
You May Like: Percent Error In Chemistry
Animal Form And Function
The cells in each animal body are bathed in interstitial fluid, which make up the cell’s environment. This fluid and all its characteristics can be described as the animal’s internal environment, which is in contrast to the external environment that encompasses the animal’s outside world. Animals can be classified as either regulators or conformers. Animals such as mammals and birds are regulators as they are able to maintain a constant internal environment such as body temperature despite their environments changing. These animals are also described as homeotherms as they exhibit thermoregulation by keeping their internal body temperature constant. In contrast, animals such as fishes and frogs are conformers as they adapt their internal environment to match their external environments. These animals are also described as poikilotherms or ectotherms as they allow their body temperatures to match their external environments. In terms of energy, regulation is more costly than conformity as an animal expands more energy to maintain a constant internal environment such as increasing its basal metabolic rate, which is the rate of energy consumption. Similarly, homeothermy is more costly than poikilothermy. Homeostasis is the stability of an animal’s internal environment, which is maintained by negative feedback loops.
Water and salt balance
Nutrition and digestion
Breathing
Circulation
What Is A Basic Definition Of Element
An element is a substance that cannot be separated into simpler substances through chemistry. An element is also an important component of something or a natural habitat. Element has many other senses as a noun.
In chemistry, an element is something that cannot be broken down any further. If you have taken a chemistry class, youve likely seen the periodic table, which displays all the known chemical elements. The study and measuring of elements is one of the central focuses of the scientific field of chemistry. For example, water is made of the elements hydrogen and oxygen. We can split water into hydrogen and oxygen, but we cannot use chemistry to split oxygen or hydrogen into anything else.
- Real-life examples: The substances we know as carbon, oxygen, nitrogen, calcium, and gold are examples of elements.
- Used in a sentence: Ammonia is made of the elements nitrogen and hydrogen.
Outside of science, an element is a main component or ingredient of something, as bricks would be for a brick wall, for example. The words elemental and elementary are sometimes used in a similar sense to describe things that are the simplest principles or basic components of something.
- Real-life examples: Peanut butter, jelly, and bread are the elements of a PB& J sandwich. Cement and water are elements of concrete. Tires, brakes, and an engine are elements of a functioning vehicle.
- Used in a sentence: Love and trust are elements of a strong relationship.
Read Also: Is Physics Hard In College
Historical Development Of The Concept Of Element
In the latter part of the Middle Ages, as alchemists became more sophisticated in their knowledge of chemical processes, the Greek concepts of the composition of matter became less satisfactory. Additional elemental qualities were introduced to accommodate newly discovered chemical transformations. Thus, sulfur came to represent the quality of combustibility, mercury that of volatility or fluidity, and salt that of fixity in fire . These three alchemical elements, or principles, also represented abstractions of properties reflecting the nature of matter, not physical substances.
The important difference between a mixture and a chemical compound eventually was understood, and in 1661 the English chemist Robert Boyle recognized the fundamental nature of a chemical element. He argued that the four Greek elements could not be the real chemical elements because they cannot combine to form other substances nor can they be extracted from other substances. Boyle stressed the physical nature of elements and related them to the compounds they formed in the modern operational way.
An Atom Often Behaves As If It Has A Fixed Radius
When a forms between two atoms, the sharing of electrons brings the nuclei of these atoms unusually close together. But most of the atoms that are rapidly jostling each other in cells are located in separate molecules. What happens when two such atoms touch?
For simplicity and clarity, atoms and molecules are usually represented in a highly schematic wayeither as a line drawing of the structural formula or as a ball and stick model. However, a more accurate representation can be obtained through the use of so-called space-filling models. Here a solid envelope is used to represent the radius of the cloud at which strong repulsive forces prevent a closer approach of any second, non-bonded atomthe so-called van der Waals radius for an atom. This is possible because the amount of repulsion increases very steeply as two such atoms approach each other closely. At slightly greater distances, any two atoms will experience a weak attractive force, known as a . As a result, there is a distance at which repulsive and attractive forces precisely balance to produce an energy minimum in each atom’s interaction with an atom of a second, non-bonded element .
The balance of van der Waals forces between two atoms. As the nuclei of two atoms approach each other, they initially show a weak bonding interaction due to their fluctuating electric charges. However, the same atoms will strongly repel each other if
You May Like: Kuta Software Simplifying Radicals Answers