What Concentration Means In Chemistry
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
In chemistry, the word “concentration” relates to the components of a mixture or solution. Here is the definition of concentration and a look at different methods used to calculate it.
What Is The Concentration In Chemistry
Concentration is a very common concept used in chemistry and related fields. It is the measure of how much of a given substance there is mixed with another substance. There exists a concentration at which no further solute will dissolve in a solution.
What is concentration in a solution?
The concentration of a solution is a measure of the amount of solute that has been dissolved in a given amount of solvent or solution. A concentrated solution is one that has a relatively large amount of dissolved solute. A dilute solution is one that has a relatively small amount of dissolved solute.
Expression Of Concentration Of Solutions
We always discuss a solution being diluted or concentrated this is a qualitative way of expressing the concentration of the solution. A dilute solution means the quantity of solute is relatively very small, and a concentrated solution implies that the solution has a large amount of solute. But these are relative terms and do not give us the quantitative concentration of the solution.
Don’t Miss: What Is Blank Solution In Chemistry
What Is A Concentrated In Science
Updated May 10, 2019. In chemistry, “concentrated” refers to a relatively large quantity of substance present in a unit amount of a mixture. Usually, this means there is a lot of a solute dissolved in a given solvent. A concentrated solution contains the maximum amount of solute that can be dissolved.
What Is A Concentration In Chemistry
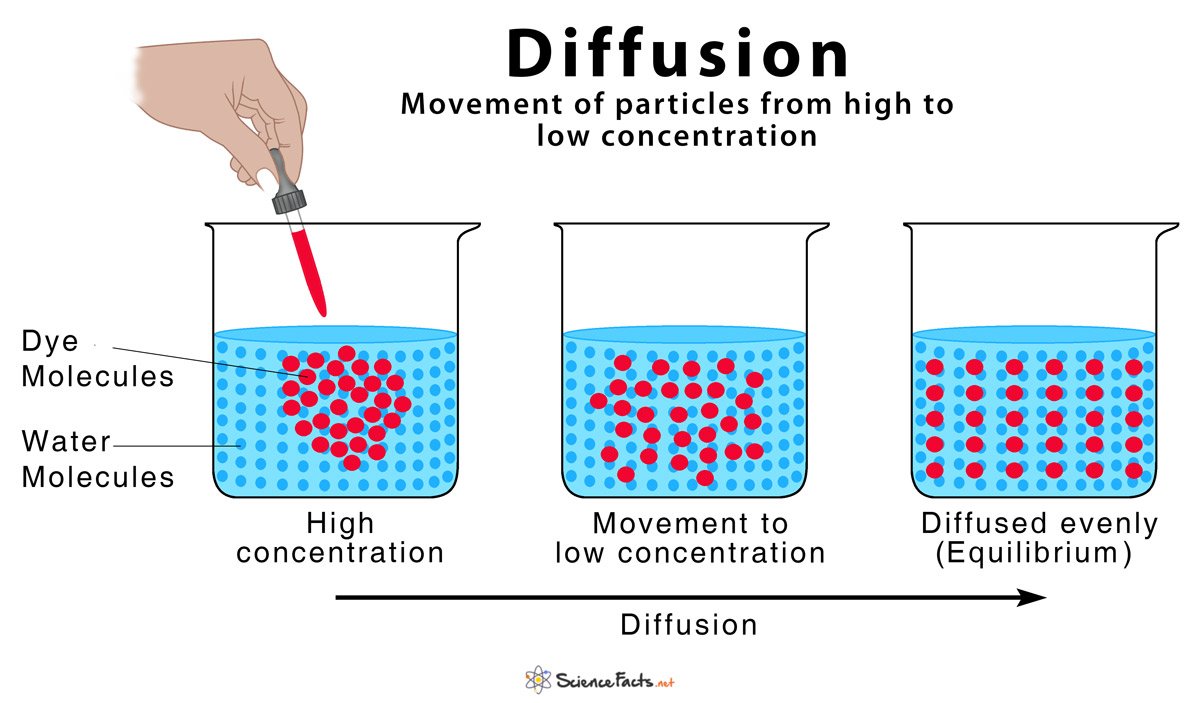
Similarly, it is asked, what does it mean by concentration of a solution?
In chemistry, concentration refers to the amount of a substance per defined space. Another definition is that concentration is the ratio of solute in a solution to either solvent or total solution. If a solution is concentrated to the point where no more solute will dissolve in the solvent, it is said to be saturated.
What is the definition of concentrated solution?
Concentrated solution is a solution that contains a large amount of solute relative to the amount that could dissolve.
Don’t Miss: What Is Entropy In Biology
How Is The Concentration Of A Solution Determined
Once you have identified the solute and solvent in a solution, you are ready to determine its concentration. Concentration may be expressed several different ways, using percent composition by mass, volume percent, mole fraction, molarity, molality, or normality.
How is the concentration ratio of an industry calculated?
The concentration ratio is calculated as the sum of the market share percentage held by the largest specified number of firms in an industry. The concentration ratio ranges from 0% to 100%, and an industrys concentration ratio indicates the degree of competition in the industry.
Which is an example of a concentration in a solvent?
Concentration refers to the amount of solute that is dissolved in a solvent. We normally think of a solute as a solid that is added to a solvent , but the solute could easily exist in another phase. For example, if we add a small amount of ethanol to water,
S Of Expressing The Concentration Of Solution
There are various methods of expressing the concentration of a solution. You will usually see Chemists working with the number of moles. Pharmacists will use percentage concentrations instead of the number of moles. Hence it is important to understand all the methods of expressing the concentration of solutions.
The concentration of the solution formula is given as follows.
Concentration of solution = \
We will also see other methods on how to calculate the concentration of a solution based on the different methods of expressing concentrations.
-
Concentration in Parts per Million
It is expressed in terms of weight. The formula for parts per million is given as follows:
ppm= \x10\
-
Mass Percentage
It is expressed in terms of mass percentage of solute to the solution. The formula for mass percentage is given as follows.
Mass percentage of A = \x100
e.g. CH3COOH 33% w/w, and H2SO4 98.0% w/w.
-
Volume Percentage
It is expressed in terms of the volume percentage of solute to the solvent. The formula for volume percentage is given as follows.
Volume percentage of A = \x100
-
Mass by Volume Percentage
Percentage weight in volume expresses the number of grams of solute in 100 ml of product.
e.g. BaCl2 solution 10% w/v, and H2O2 solution 5-7% w/v.
-
Molarity
It is the number of moles of solute contained in 1000 ml of solution. It is a commonly used method for expressing concentrations.
Molarity = \
-
Molality
Molality = \
-
Normality
Normality = \
-
Mole Fraction:
X\ = \
X\ = \
Don’t Miss: How To Find Distance In Physics
An Introduction To Concentration Of Solution
Everyone talks about the concentration of solutions. They may also talk about the concentration of coffee or tea. Everyone has a particular view of what is meant by the concentration of a solution. You must have noticed that whenever you make coffee, if you add a lot of powder, you will end up with a concentrated drink, whereas if you add little, it will result in a dilute solution. Therefore, it is essential that you understand what the concentration of the solution is. In this chapter, we will learn about what is meant by the concentration of a solution we will also see how to find the concentration of a solution and the different methods of expressing the concentration of the solution.
Chemistry Is Everywhere: Preparing Iv Solutions
In a hospital emergency room, a physician orders an intravenous delivery of 100 mL of 0.5% KCl for a patient suffering from hypokalemia . Does an aide run to a supply cabinet and take out an IV bag containing this concentration of KCl?
Not likely. It is more probable that the aide must make the proper solution from an IV bag of sterile solution and a more concentrated, sterile solution, called a stock solution, of KCl. The aide is expected to use a syringe to draw up some stock solution and inject it into the waiting IV bag and dilute it to the proper concentration. Thus the aide must perform a dilution calculation.
If the stock solution is 10.0% KCl and the final volume and concentration need to be 100 mL and 0.50%, respectively, then it is an easy calculation to determine how much stock solution to use:
V1 = V1 = 5 mL
Of course, the addition of the stock solution affects the total volume of the diluted solution, but the final concentration is likely close enough even for medical purposes.
Medical and pharmaceutical personnel are constantly dealing with dosages that require concentration measurements and dilutions. It is an important responsibility: calculating the wrong dose can be useless, harmful, or even fatal!
You May Like: What Comes After Geometry In High School
Why Is Concentration Important In Chemistry
The concentration of a solute is very important in studying chemical reactions because it determines how often molecules collide in solution and thus indirectly determines the rates of reactions and the conditions at equilibrium . Concentration may be expressed in a number of ways.
What is meant by absolute concentration?
For example, if I dissolved 20 g of NaCl in a total volume of 100 mL of water I would say that I have a 0.2 g/mL solution of NaCl. This would be an absolute concentration because it tells me exactly how much NaCl is in a particular volume of solution.
What is the concept of concentration?
The concentration of a substance is the quantity of solute present in a given quantity of solution. Concentrations are usually expressed in terms of molarity, defined as the number of moles of solute in 1 L of solution.
How Do You Prepare A Solution Of Known Concentration
Solutions of known concentration can be prepared either by dissolving the known mass of the solvent solution and diluting it to the desired final volume or by diluting it to the desired final volume by diluting the acceptable volume of the more concentrated solution .
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
Don’t Miss: How Do You Do Average In Math
How To Calculate Concentration
Concentration is determined mathematically by taking the mass, moles, or volume of solute and dividing it by the mass, moles, or volume of solution . Some examples of concentration units and formulas include:
- Molarity – moles of solute/liters of solution
- Mass Concentration – mass of solute/volume of solution
- Normality – grams active solute/liters of solution
- Molality – moles of solute/mass of solvent
- Mass Percent – mass solute/mass solution x 100%
- Volume Concentration – volume of solute/volume of mixture
- Number Concentration – number of entities of a component divided by the total volume of the mixture
- Volume Percent – volume solute/volume solution x 100%
- Mole Fraction – moles of solute/total moles of species in the mixture
- Mole Ratio – moles of solute/total moles of all other species in the mixture
- Mass Fraction – mass of one fraction /total mass of the mixture
- Mass Ratio – mass of solute/mass of all other constituents in the mixture
- PPM – a 100 ppm solution is 0.01%. The “parts per” notation, while still in use, has largely been replaced by mole fraction
- PPB – typically used to express contamination of dilute solutions
Some units may be converted from one to another. However, it’s not always a good idea to convert between units based on the volume of solution to those based on mass of solution because volume is affected by temperature.
Strength Of A Solution
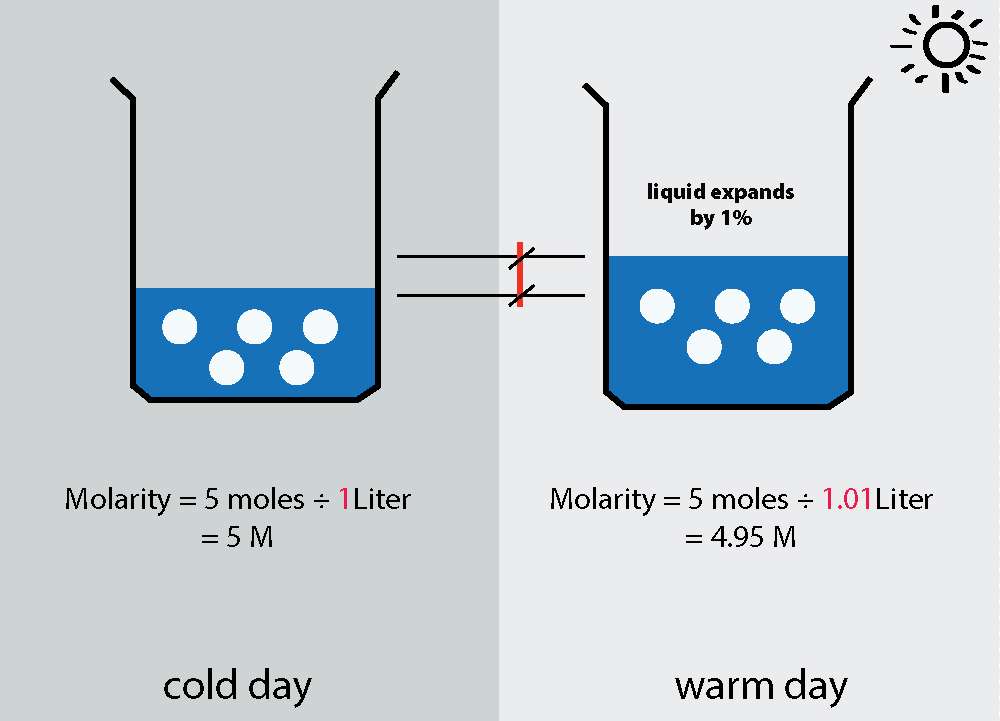
We need a way to represent the relative amount of solute dissolved in a solvent.
It makes sense that some solutions are stronger than others: adding a tiny bit of acid to a gallon of water, for example, might not even be detectable. But add a lot of acid and you have a dangerous solution that needs to be handled carefully.
We need a way to represent the relative strength of a solution. We’ll call it concentration.
There are several methods of concentration measurement, each used in different kinds of situations.
Read Also: Does Nicole Kidman Have Biological Children
What Is The Concentration Of Naoh That Is Used
To make 1 N solution, dissolve 40.00 g of sodium hydroxide in water to make volume 1 liter. For a 0.1 N solution 4.00 g of NaOH per liter is needed.
What best describes the concentration of a solution?
The concentration of a solution is a measure of the amount of solute that has been dissolved in a given amount of solvent or solution. A concentrated solution is one that has a relatively large amount of dissolved solute. A dilute solution is one that has a relatively small amount of dissolved solute.
Which is the correct definition of a concentration?
What is concentration? Concentration refers to the amount of solute that is dissolved in a solvent. We normally think of a solute as a solid that is added to a solvent , but the solute could easily exist in another phase.
Is Fresh Orange Juice Better Than Concentrate
Go for fresh OJ as a better source of vitamins than orange juice from concentrate. … Juice from concentrate offers an economical alternative to fresh juices and provides some nutritional value, but it loses some of its nutrients during processing. Both types of juice have a place in a balanced diet.
Also Check: What Is Paramagnetic And Diamagnetic In Chemistry
Solutions Of Solids In Liquids
- A saturated solution is a solution that remains in contact with an excess of solute.
- The amount of solute dissolved per 100g of solvent in a saturated solution at a specific temperature represents the solubility of the solute.
- For exothermic substances such as KOH, CaO, Ca2, M2CO3, M2SO4, etc, solubility is inversely proportional to temperature.
- For endothermic substances such as NaCl, KNO3, NaNO3, glucose, etc., solubility is directly proportional to temperature.
What Is Concentration Of A Solution
In an aqueous solution, two parts exist, namely solute and solvent. They are the two basic solution concentration terms that you need to know. We always need to keep an account of the amount of solute in the solution. In chemistry, we define the concentration of solution as the amount of solute in a solvent. When a solution has more solute in it, we call it a concentrated solution. Whereas when the solution has more solvent in it, we call it a dilute solution.
Now that you understand the concept of what is the concentration of solution let’s move on to the different methods of expressing concentration.
You May Like: What Does Urban Mean In Geography
What Do You Mean By Molality
Molality definition Molality , or molal concentration, is the amount of a substance dissolved in a certain mass of solvent. It is defined as the moles of a solute per kilograms of a solvent.
What is concentration of data?
What is concentration data? Concentration data is a document in which all the concentration of individual time points Concentration data is used to calculate pharmacokinetics parameters and statistical parameters for bioequivalence calculation of a pharmaceutical generic formulation.
How to calculate concentration?
Method 1 of 3: Using the Mass per Volume Equation. Find the mass of the solute mixed in with the solvent.
What is the importance of concentration?
Importance of Concentration in Vocations. Concentration is a requirement of success in vocation.
What Does Relative Concentration Mean
Concentrated and dilute are qualitative terms that reflect the relative concentration of a solution, which is the amount of solute dissolved in a given amount of solvent. The mass percent of a solution is the mass of the solute divided by the mass of the solution.
What is concentration used for?
The concentration of a chemical substance expresses the amount of a substance present in a mixture. There are many different ways to express concentration. Chemists use the term solute to describe the substance of interest and the term solvent to describe the material in which the solute is dissolved.
What is the difference between absolute and relative concentration?
The absolute O2 concentration determines the rate of most biological and chemical processes, but the relative O2 concentration is typically reported. This is analogous to discussing relative humidity when absolute humidity is what determines evaporation rates.
You May Like: Using Sacred Geometry To Manifest
Using The Mass Per Volume Equation
Tip: If you need to use a scale, subtract the mass of the container youre using to hold the solute or else your calculations will be off.
What Is The Concentration Of A Solution
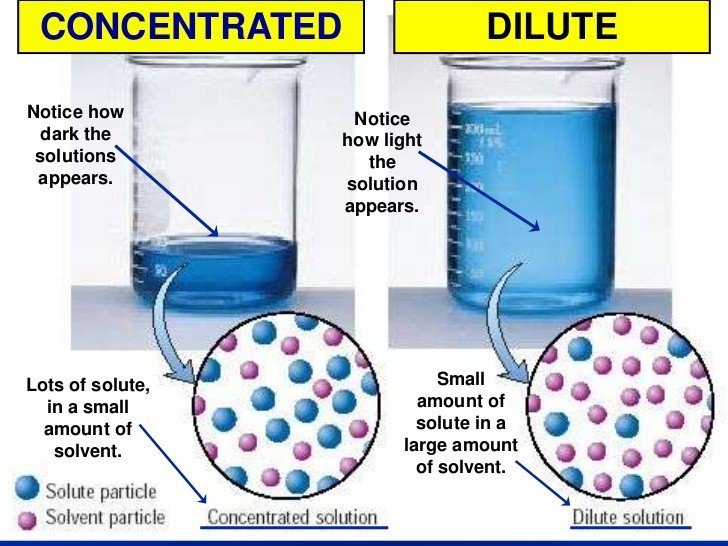
A solution concentration is a measure of the quantity of solute that has been dissolved in a given quantity of solvent or solution. One that contains a relatively high volume of dissolved solute is a concentrated solution. That that contains a relatively minimal volume of dissolved solute is a dilute solution.
Also Check: How Did Sigmund Freud Contribute To The Field Of Psychology
Normal Concentration Definition In Chemistry
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
There are two meanings for “normal” in chemistry. Normal or normal concentration refers to a concentration of solutes that is the same in two samples. Normality is the gram equivalent weight of a solution in a solution, which is its molar concentration divided by an equivalence factor. It is used in situations where molarity or molality might be confusing or else difficult to determine. Normal concentration is also known as normality, N, isotonic.