Chapter 1: The Mole And Molar Mass
- To calculate the molecular mass of a covalent compound.
- To calculate the formula mass of an ionic compound.
- To calculate the number of atoms, molecules or formula units in a sample.
Chemistry is the study of how atoms and molecules interact with each other which occurs on the atomic scale. Chemists need a way of simply determining how many molecules they have in a beaker. The mole concept, which we will introduce here, bridges that gap by relating the mass of a single atom or molecule in amu to the mass of a collection of a large number of such molecules in grams.
Finding Average Mr Of A Gaseous Mixture
A 2 g mixture of cyclohexanol, acetone and pentanal is completely burnt in oxygen. When the gaseous product is passed through $\ce$, the mass of $\ce$ increased by 1.998 g. Find the average molecular mass of the mixture.
$\ce$ absorbs water, so the amount of water absorbed is 0.111 mol. I attempted constructing an equation for the combustion reaction but realized that the proportions and thus the mole ratios of each gas are unknown.
- Chet MillerOct 19, 2017 at 1:05
- $\begingroup$Are you asking about the average relative molecular mass $M_\mathrm r$ , the average molecular mass , or the average molar mass $M$ ? These are different quantitites with different dimensions.$\endgroup$ user7951
I suspect one might utilize the fact that all three compounds cyclohexanol $\ce$, acetone $\ce$ and pentanal $\ce$ share the same empirical formula $\ce$, which is also applicable to the mixture of reactants, and so instead of writing a set reaction equations
\begin\ce \tag\\\ce \tag\\\ce \tag\end
one can avoid finding volume fraction $\phi_i$ of each individual component and use a single reaction scheme such as
$$\ce$ O2 -> x H2O + x CO2} \tag$$
and find relation between average molecular weight $\bar$ and $x$ solely from . Lets denote the mass and the total amount of gas mixture components as $m$ and $n$, respectively. Then according to total amount of gas mixture is $n = \frac)}$, and the average molecular weight is established as
$$\bar = x \cdot + 2M) + M = \,\pu \tag$$
Worked Examples Of Calculating Mass Moles Molar Mass
In each of the worked examples below, you will be asked to calculate either the moles, mass, or molar mass of a pure substance.
To answer each question correctly you will need to:
Don’t Miss: Introduction To Rational Functions Common Core Algebra 2 Homework Answers
What Is Relative Isotopic Mass
In nature, two of the same atoms can exist but have a different number of neutrons.
When an atom of the same element has a different number of neutrons, it is called an isotope.
The mass of an atom of an isotope compared to of the mass of carbon-12 is called relative isotopic mass.
To calculate the relative isotopic mass scientists use this formula:
Scientists measure the mass of an atom of an isotope by comparing it to one unified atomic mass unit or 1u. 1u equals of the mass of a carbon-12 atom.
Note: You wont use this formula in your exam but its good to know!
Calculating The Molar Mass Of An Element
Don’t Miss: How Physics Is Related To Other Branches Of Science
Problem Solving Using Moles Mass And Molar Mass
The Problem:
Chris the Chemist has an impure sample of calcium carbonate.The mass of the impure sample is 0.1250 kg and it is composed of 87.00% calcium carbonate.Before Chris can use this calcium carbonate in a chemical reaction, Chris needs to know the amount, in moles, of calcium carbonate present in this sample.
Calculate the amount of calcium carbonate in moles present in this impure sample of calcium carbonate.
Solving the Problem using the StoPGoPS model for problem solving:
| STOP! |
Are You Experiencing Difficulty Reading Text On This Site Or Downloaded From This Site
- Click on this link for more information on using the magnifier on a Windows PC:
- Click on this link for more information on using the magnifier on a Mac computer
- Click on this link for more information on using the free, open source Orca magnifier software on a computer running Linux:
You May Like: What Is Inertia In Physics
Worked Example: Moles = Mass ÷ Molar Mass
Question: Calculate the amount of oxygen gas, O2, in moles present in 124.5 g of oxygen gas.
Solution:
Step 1. Extract the data from the question:
mass = m = 124.5 g
moles = n = ? mol
Step 2. Check the data for consistency:
Is the mass of oxygen gas in grams ? Yes. We do not need to convert this.
Step 3. Write the mathematical equation :
moles = mass ÷ molar mass or n = m ÷ M
Step 4. Substitute the values into the equation and solve to find moles of oxygen gas:
moles = n = 124.5
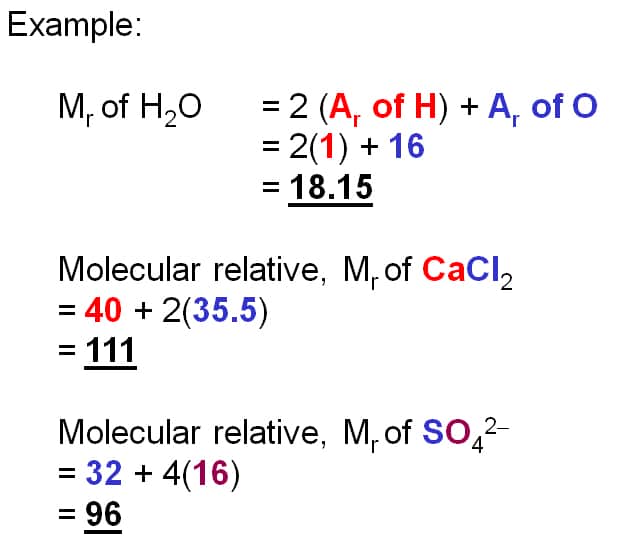
Relative Formula Mass& Relative Molecular Mass
- We have seen previously that the symbol for the relative atomic mass is Ar
- This is calculated from the mass number and relative abundances of all the isotopes of a particular element
- The symbol for the relative formula mass is Mr and it refers to the total mass of the substance
- If the substance is molecular you can use the term relative molecular mass, but this term should not be used for ionic compounds such as sodium chloride
- To calculate the Mr of a substance, you have to add up the relative atomic masses of all the atoms present in the formula
Relative Formula Mass Calculations Table
- In accordance with the Law of Conservation of Mass, the sum of the relative formula masses of the reactants will be the same as the sum of the relative formula masses of the products
Exam Tip
If you are in any doubt whether to use relative molecular mass or relative formula mass, use the latter because it applies to all compounds whether they are ionic or covalent.
Calculating percentage by mass of an element in a compound
- The percentage by mass of an element in a compound can be calculated using the following equation:
Worked Example
Calculate the percentage by mass of calcium in calcium carbonate, CaCO3
Answer
Exam Tip
Dont forget to multiply your answer by 100 in order to convert it to a percentage.
Don’t Miss: What Is Eq In Psychology
Calculating The Molar Mass Of A Compound
Formula Mass And Mole Calculations
The relative formula mass of a compound is calculated by adding together the relative atomic mass values for all the atoms in its formula. Moles are units used to measure substance amount.
This can be rearranged to find the mass if the number of moles and molar mass are known. It can also be rearranged to find the molar mass if the mass and number of moles are known.
The triangle diagram may help you with this.
Also Check: What Is Biological Monitoring In Ecosystems
What Is Relative Molecular Mass
The weighted average of the mass of a molecule relative to 112 of the mass of a carbon-12 atom is called the relative molecular mass .
We must say ‘weighted average’ when speaking about RMM. As an example, let’s look at the molecule .
An average sample of molecules will have both chlorine-37 and chlorine-35 atoms. This means that the masses of the molecules will vary, like this:
12 + 1 + = 118
12 + 1 + + 37 = 120
12 + 1 + 35 + = 122
12 + 1 + = 124
So a weighted average includes how many of each of these molecules we find in an average sample of a substance. We calculate the abundance of an isotope as a percentage.
Relative molecular mass refers to molecules with a fixed number of atoms joined together by covalent bonding, including noble gases. It does not include things ionically bonded together like sodium chloride .
We can calculate Mr by adding up the relative masses of the atoms in a molecule. For example, the molecule has two hydrogen atoms and one oxygen atom. You can calculate its molecular mass like this:
Olive , StudySmarter Originals
Calculations For All Students
An empirical formula of a substance is found using the masses and relative atomic masses of the elements it contains. The law of conservation of mass applies to closed and non-enclosed systems.
so instead of using their actual masses in kilograms, their relative atomic masses are used. The relative atomic mass of an element, symbol Ar, is defined as the relative mass of its atoms compared to the mass of a particular carbon atom . The Ar values for elements are often stated in the periodic table. Note, as Ar is a measure of the relative masses of atoms, it has no units.
The relative atomic masses of elements are proportional measures. For example, the Ar for carbon is 12, and the Ar for magnesium is 24. This means that magnesium atoms are twice the mass of carbon atoms.
Don’t Miss: When To Round In Nursing Math
Example Of Simple Molecular Mass Calculation
For example, to find the molecular mass of NH3, the first step is to look up the atomic masses of nitrogen and hydrogen .
H = 1.00794N = 14.0067
Next, multiply the atomic mass of each atom by the number of atoms in the compound. There is one nitrogen atom . There are three hydrogen atoms, as indicated by the subscript.
molecular mass = + molecular mass = 14.0067 + 3.02382molecular mass = 17.0305
Note the calculator will give an answer of 17.03052, but the reported answer contains fewer significant figures because there are six significant digits in the atomic mass values used in the calculation.
Calculating The Moles Of A Pure Substance
In the discussion above, we discovered that we could calculate the mass of a pure substance using the moles and molar mass of the substance:
mass = moles × molar mass
How would we calculate the moles of pure substance if we knew the mass of the substance?
We could use some algebra: divide both sides of the equation by the molar mass:
| mass |
moles = mass ÷ molar mass
n = m ÷ M
Follow these steps to calculate the amount of pure substance in moles given the mass of substance:
Step 1. Extract the data from the question:
mass = m = write down what you are told in the question
moles = n = ?
molar mass = M = write down what you are told in the question
Step 2. Check the units for consistency and convert if necessary:
Mass must be in grams !
If mass is given in milligrams , divide it by 1,000 to give the mass in grams .
If mass is given in micrograms , divide it by 1,000,000 to give a mass in grams .
If mass is given in kilograms , multiply it by 1,000 to give a mass in grams .
Step 3. Write the mathematical equation :
moles = mass ÷ molar mass
n = m ÷ M
M = m ÷ n
or We could use some logic:
Therefore, molar mass = mass ÷ moles
or you can write
M = m ÷ n
Follow these steps to calculate the molar mass of a pure substance given the amount of substance in moles and the mass of substance:
Step 1. Extract the data from the question:
mass = m = write down what you are told in the question
moles = n = write down what you are told in the question
molar mass = M = ?
M = m ÷ n
Don’t Miss: What Does Human Development Index Mean In Geography
Worked Example: Mass = Moles Molar Mass
Question: Calculate the mass of 0.25 moles of water, H2O.
Solution:
Step 1. Extract the data from the question:
moles = n = 0.25 mol
mass = m = ? g
Step 2. Check the data for consistency:
Is the amount of water in moles ? Yes. We do not need to convert this.
Step 3. Write the mathematical equation :
mass = moles × molar mass
Creating The Gas Bags
Note: wear eye protection.
The gas bags should be filled shortly before the lesson to minimise any losses by leakage. A selection from the following would be suitable, preferably from gas cylinders or the laboratory gas supply :
Note
Oxygen and carbon dioxide are also available in small pressurised canisters. However, the purity of the gases in these cylinders, and of methane from the laboratory natural gas supply, may be significantly less than 100%, which will affect the RMM value obtained.
Also Check: Who Is Known As The Father Of Nuclear Physics
Calculating Molecular Formulas For Compounds
- Understand the difference between empirical formulas and molecular formulas.
- Determine molecular formula from percent composition and molar mass of a compound.
Below, we see two carbohydrates: glucose and sucrose. Sucrose is almost exactly twice the size of glucose, although their empirical formulas are very similar. Some people can distinguish them on the basis of taste, but it’s not a good idea to go around tasting chemicals. The best way to tell glucose and sucrose apart is to determine the molar massesâthis approach allows you to easily tell which compound is which.