Rules For Observing The Type Of Hybridisation
The following rules are observed to understand the type of hybridisation in a compound or an ion.
- Calculate the total number of valence electrons.
- Calculate the number of duplex or octet OR
- Number of lone pairs of electrons
- Number of used orbital = Number of duplex or octet + Number of lone pairs of electrons
- If there is no lone pair of electrons then the geometry of orbitals and molecule is different.
Examples Of Sp3d Hybridization
In sp3d hybridized orbitals, s orbital, three p orbitals and one d orbital are hybridized. When atom has sp3d hybridization, summation of number of sigma bonds and number of lone pairs around that atom should be 5.
Because all p orbitals are hybridized, y = 3. Therefore we can find the z from our equation.
- Summation of number of sigma bonds and number of lone pairs around an atom = x + y + z = 5
- 1 + 3 + z = 5
- z = 1
Assignment Of Hybrid Orbitals To Central Atoms
The hybridization of an atom is determined based on the number of regions of electron density that surround it. The geometrical arrangements characteristic of the various sets of hybrid orbitals are shown in Figure 16. These arrangements are identical to those of the electron-pair geometries predicted by VSEPR theory. VSEPR theory predicts the shapes of molecules, and hybrid orbital theory provides an explanation for how those shapes are formed. To find the hybridization of a central atom, we can use the following guidelines:
Figure 16.spsp
Don’t Miss: Grade 6 Fsa Warm Ups Answer Key
Sp3d And Sp3d2 Hybridization
To describe the five bonding orbitals in a trigonal bipyramidal arrangement, we must use five of the valence shell atomic orbitals , which gives five sp3d hybrid orbitals. With an octahedral arrangement of six hybrid orbitals, we must use six valence shell atomic orbitals , which gives six sp3d2 hybrid orbitals. These hybridizations are only possible for atoms that have d orbitals in their valence subshells .
In a molecule of phosphorus pentachloride, PCl5, there are five PCl bonds directed toward the corners of a trigonal bipyramid. We use the 3s orbital, the three 3p orbitals, and one of the 3d orbitals to form the set of five sp3d hybrid orbitals that are involved in the PCl bonds. Other atoms that exhibit sp3d hybridization include the sulfur atom in SF4 and the chlorine atoms in ClF3 and in ClF4+.
Figure 13.spdFigure 14.spd
The sulfur atom in sulfur hexafluoride, SF6, exhibits sp3d2 hybridization. A molecule of sulfur hexafluoride has six bonding pairs of electrons connecting six fluorine atoms to a single sulfur atom. There are no lone pairs of electrons on the central atom. To bond six fluorine atoms, the 3s orbital, the three 3p orbitals, and two of the 3d orbitals form six equivalent sp3d2 hybrid orbitals, each directed toward a different corner of an octahedron. Other atoms that exhibit sp3d2 hybridization include the phosphorus atom in PCl6, the iodine atom in the interhalogens IF6+, IF5, ICl4, IF4 and the xenon atom in XeF4.
Figure 15.spdspd
Formation Of Nh3 And H2o Molecules
In NH2 molecule nitrogen atom is sp3-hybridised and one hybrid orbital contains two electrons. Now three 1s- orbitals of three hydrogen atoms overlap with three sp3 hybrid orbitals to form NH3 molecule. The angle between H-N-H should be 109.50 but due to the presence of one occupied sp3-hybrid orbital the angle decreases to 107.80. Hence, the bond angle in NH3 molecule is 107.80.
Don’t Miss: How To Calculate Half Life From Rate Constant
Molecules With Lone Pairs
For molecules with lone pairs, the bonding orbitals are isovalent spx hybrids. For example, the two bond-forming hybrid orbitals of oxygen in water can be described as sp4.0 to give the interorbital angle of 104.5°. This means that they have 20% s character and 80% p character and does not imply that a hybrid orbital is formed from one s and four p orbitals on oxygen since the 2p subshell of oxygen only contains three p orbitals. The shapes of molecules with lone pairs are:
In such cases, there are two mathematically equivalent ways of representing lone pairs. They can be represented by orbitals of sigma and pi symmetry similar to molecular orbital theory or by equivalent orbitals similar to VSEPR theory.
Hypervalent molecules
For hypervalent molecules with lone pairs, the bonding scheme can be split into a hypervalent component and a component consisting of isovalent spx bond hybrids. The hypervalent component consists of resonant bonds using p orbitals. The table below shows how each shape is related to the two components and their respective descriptions.
| Number of isovalent bond hybrids |
|---|
| Two |
Identifying Hybridization In Molecules
Figuring out what the hybridization is in a molecule seems like it would be a difficult process but in actuality is quite simple. Because hybridiztion is used to make atomic overlaps, knowledge of the number and types of overlaps an atom makes allows us to determine the degree of hybridization it has. In other words, you only have to count the number of bonds or lone pairs of electrons around a central atom to determine its hybridization.
The following rules give the hybridization of the central atom: 1 bond to another atom or lone pair = s 2 bonds to another atom or lone pairs = sp 3 bonds to another atom or lone pairs = sp2 4 bonds to another atom or lone pairs = sp3 5 bonds to another atom or lone pairs = sp3d 6 bonds to another atom or lone pairs = sp3d2
This Video Explains it further:
Practice Example:
Don’t Miss: Algebra 2 Radical Worksheet
What Is Hybridization Of No+
In nitrogen is sp hybridized, one sp hybrid orbital is used to bond and remaining holds lone pair of electrons and then two p orbitals can be used form two bonds with oxygen atom. In nitrogen is hybridized, one hybrid orbital is used to bond with oxygen and remaining two hybrid orbitals hold two electron pairs.
How To Calculate Hybridization
Electrons revolve around their atoms in orbits. In valence bond theory, atomic orbitals of one atom can overlap with the orbitals of other atoms to form a molecule, creating brand new, hybrid orbitals. This phenomenon is known as hybridization. Determining the hybridization of a molecule can help identify its shape and structure. For example, many molecules settle in a shape that minimizes the amount of repulsion between atoms and electrons, creating a shape that requires as little energy as possible to maintain. Knowing the types of shapes a molecule will take when hybridized helps researchers better understand how that molecule may interact with others. Hybridization affects the types of bonds that a molecule can make.
You May Like: Is Physics Harder Than Math
Localized Vs Canonical Molecular Orbitals
Bonding orbitals formed from hybrid atomic orbitals may be considered as localized molecular orbitals, which can be formed from the delocalized orbitals of molecular orbital theory by an appropriate mathematical transformation. For molecules in the ground state, this transformation of the orbitals leaves the total many-electron wave function unchanged. The hybrid orbital description of the ground state is, therefore equivalent to the delocalized orbital description for ground state total energy and electron density, as well as the molecular geometry that corresponds to the minimum total energy value.
Explanation Of Hybridization Through Examples
Example 1: Consider an example of the simplest hydrocarbon molecular Methane. CH. According to experimental observations, the Methane molecule has 4 identical C-H bonds with equal length and equal bond energy. All the four hydrogen atoms are arranged in a manner such that the four hydrogen atoms form corners of a regular tetrahedron.
Image: Structural Formula of Methane
Based on the valence theory, a covalent bond is formed between two atoms in a molecule when there is an overlapping of half-filled atomic orbitals containing unpaired electrons. In the case of the methane molecule, we first write down the electronic configuration of each atom – C and H
Image: Electronic configuration of carbon and hydrogen for hybridization
Each carbon atom has two unpaired electrons . Based on the valence theory, only two hydrogen molecules could be paired to the two unpaired electrons of the carbon atom and there will be a formation of only 2 C-H bonds in the molecule. This will lead to an incomplete octet in the 2nd orbital of the carbon molecule and so the molecule should be unstable. However, we see that actually the methane molecule is extremely stable in nature and has 4 C-H bonds and not two. Thus, the valence theory doesnt explain the covalent bond of the methane molecule.
The hybridization concept explains the formation of identical 4 C-H bonds and the tetrahedral shape of the molecule.
Image: Electronic configuration of carbon in the ground state and in the excited state
You May Like: What Is The Shape Of Ccl4
A Solved Question For You
Q: Discuss the rules of hybridisation. Are they important to the study of the concept as a whole?
Ans: Yes, the rules of hybridisation are very important to be studied before diving into the subject of hybridisation. Hence, these rules are essential to the understanding of the concepts of the topic. The following are the rules related to hybridisation:
- Orbitals of only a central atom would undergo hybridisation.
- The orbitals of almost the same energy level combine to form hybrid orbitals.
- The numbers of atomic orbitals mixed together are always equal to the number of hybrid orbitals.
- During hybridisation, the mixing of a number of orbitals is as per requirement.
- The hybrid orbitals scattered in space and tend to the farthest apart.
- Hybrid bonds are stronger than the non-hybridised bonds.
When you once use an orbital to build a hybrid orbital it is no longer available to hold electrons in its pure form. You can hybridize the s and p orbitals in three ways.
How Do You Determine Sp Sp2 And Sp3

1:023:35How to Determine the Hybridization of an Atom (sp, sp2, sp3, sp3d, sp3d2 …YouTubeStart of suggested clipEnd of suggested clipIt. Would have one two three four electron groups so it’s hybridization is also sp3 this carbon isMoreIt. Would have one two three four electron groups so it’s hybridization is also sp3 this carbon is attached to one. Two three groups. So it’s hybridization is sp2.
Read Also: Homework 2 Segment Addition Postulate Answer Key
How Can I Calculate Hybridization Of An Atom Class 11 Chemistry Cbse
How can I calculate hybridization of an atom.
VerifiedHint: Hybridization is understood to be the idea of mixing two atomic orbitals which have a similar degree of energy which further gives two degenerate new orbitals. The intermixing is dependant on quantum mechanics. Complete answer: Hybridization is essentially in line with the basis mixing of hybrid orbitals which is classified into following types:1. $sp$ hybridization: One s orbital is combined with one p orbital and forms new equivalent atomic orbitals which have straight line shape. 2. $s^$hybridization: when one s and 2 p orbitals of the identical covering of the atom are mixed together to create 3 equivalent orbits. 3. $s^$hybridization: When one s orbital and three p orbitals of the atom mixed together to create four new equivalent orbits, then this kind of hybridization is called tetrahedral hybridization. 4. $s^d$hybridization: Within this type 3p orbitals combined with d orbital and form five $s^d$ hybridized orbitals of equal energy and also have trigonal bipyramidal geometry. 5.
Video advice: How to determine Hybridization s, sp, sp2, and sp3 Organic Chemistry
This video is about figuring out how to determine the hybridization of each element in its structure. Orbital hybridization is the concept of mixing atomic orbitals into new hybrid orbitals suitable for the pairing of electrons to form chemical bonds in valence bond theory.
Video advice: EASY Method to Find the Hybridization of an Atom
How To Find Hybridization Of Central Atom & Shape Of Molecule
Many students face problems with finding the hybridization of given atom in a compound and the shape of molecule. Nevertheless, it is very easy to determine the state of hybridization and geometry if we know the number of sigma bonds and lone pairs on the given atom. On this page, I am going to explain you how to determine them in 5 easy steps.
If you are not sure …..What is Hybridization in chemistry?….Watch the following video
Don’t Miss: Cc2 Selected Answers Chapter 7
How Do We Determine Hybridization
The easiest way to determine hybridization is to with the VSEPR theory and determine the number of electron groups around your central atom. To put it plain, I can summarize the hybridizations in the following picture:
So, the 3 groups around the central atom gives you the sp3 hybridization, the three groups gives you sp2 hybridization, and the two groups yield the sp-hybridized species. Also remember, we do count the the spare electron pairs as the electron groups too!
Unless the electron pair is next to a double or a triple bond , the electron pair will be on the hybrid orbital and not the p-orbital. While that is not 100% true in reality, thats the way we treat it within the scope of a typical organic chemistry class, so well stick with it too.
Hybridization Of Phosphorus Pentachloride
There are only five sigma bonds around the phosphorus atom in PCl5 .
Questions
In nitrogen trichloride, there are one lone pair and three N-Cl bonds around nitrogen bonds. Therefore, summation of number of lone pairs and sigma bonds around is four. Therefore, hybridization of nitrogen atom should be sp3.
Answer is 3
Recommended Reading: John Thomas Child Of Rage
Some Simple Worked Examples Of The Hybridization Shortcut
sp3 hybridization: sum of attached atoms + lone pairs = 4
sp2 hybridization: sum of attached atoms + lone pairs = 3
sp hybridization: sum of attached atoms + lone pairs = 2
Where it can start to get slightly tricky is in dealing with line diagrams containing implicit hydrogens and lone pairs. Chemists like time-saving shortcuts just as much as anybody else, and learning to quickly interpret line diagrams is as fundamental to organic chemistry as learning the alphabet is to written English.
Remember:
- Just because lone pairs arent drawn in on oxygen, nitrogen, and fluorine doesnt mean theyre not there.
- Assume a full octet for C, N, O, and F with the following one exception: a positive charge on carbon indicates that there are only six electrons around it. .
Formation Of The Hybridized Orbitals
Ok, now when we know that hybridization is a model and not an actual process, lets look at how this process happens. Each bond takes 2 electrons to complete. If we look at the carbon atom atomic orbitals, well see the 2 electrons on the 2s and 2 electrons on the 2p shells. This would only allow carbon to make 2 bonds since it only has 2 unpaired electrons. To make four bonds, carbon would have to decouple its s-electrons onto the p shell.
Rearranging the electrons in an atom in this way also makes the orbitals closer in energy making them virtually degenerate. This allows for easier mixing or hybridization as we know it. Thus, when we mix those orbitals together we end up with a set of hybrids and any leftovers that were not hybridized.
An important thing to remember: # of AOs = # of MO. So, when we mix the atomic orbitals to make the hybrids, we will end up with the exactly the same number of the the orbitals when were done. Thus, by mixing 4 orbitals , well always get 4 molecular orbitals .
Don’t Miss: The Founder Of Behaviorism Was
What Is Sp Hybridization Example
Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. Some examples include the mercury atom in the linear HgCl2 molecule, the zinc atom in Zn2, which contains a linear CZnC arrangement, the carbon atoms in HCCH and CO2, and the Be atom in BeCl2.
Exception #2 Geometric Constraints

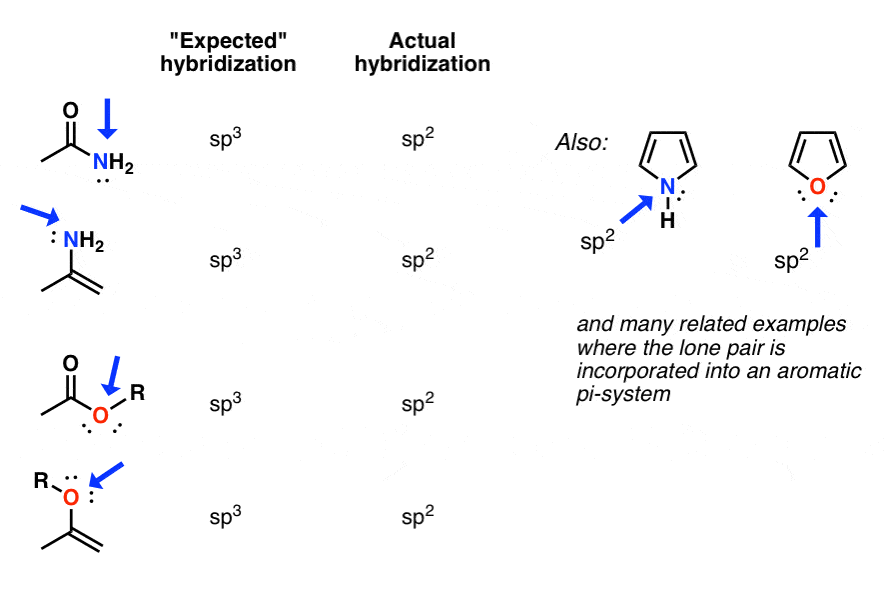
Another example where the actual hybridization differs from what we might expect from the shortcut is in cases with geometric constraints. For instance in the phenyl cation below, the indicated carbon is attached two two atoms and zero lone pairs. Whats the hybridization?
From our shortcut, we might expect the hybridization to be sp.
In fact, the geometry around the atom is much closer to sp2. Thats because the angle strain adopting the linear geometry would lead to far too much angle strain to be a stable molecule.
Also Check: What Influence Did Geography Have On The Development Of Greek Society
How Do We Find Hybridization When Molecule Has Resonance
Consider the following image which shows the resonance structures of $\ce}$ ion. What is the hybridization of $\ce$? How do we proceed in such cases? In one resonance structure, the top oxygen has $\ce$ hybridization and in others, it has $\ce$ hybridization. In the hybrid structure, shown in the second image, I don’t even know how to proceed.
- 2$\begingroup$All the oxygens should be considered to be sp² hybridised, with its resonance-contibuting p orbitals being unhybridised and acting solely as pi-bonds.$\endgroup$
- 4
The only important thing to consider for the description in terms of hybridisation is the molecular structure. It is therefore irrelevant if you are using a resonance structure, all resonance structures, or the resonance hybrid, or even some kind of completely different diagram. The structure for all of these is the same.
Therefore, to determine a reasonable hybridisation scheme, you should first look at local coordination of the atom. Please keep in mind that the $x$ in $\mathrm^x$ doesn’t need to be an integer value.For this, please look up Bent’s rule and Coulson’s theorem. You may start with my answer here: How is Bent’s rule consistent with LCAO MO theory?Please also keep then in mind that only orbitals hybridise and any combination of hybrid orbitals my be a valid description.*
If you keep this in mind, the following definition makes quite a bit more sense, hybridization in the Gold Book
Footnotes: