How Much Space Does A Mole Occupy
Now just how many is 6.02 x 10²³? How long do you think it would take you to count to a mole? One day? One week? One year? Go ahead, start counting. It would take you around 20,000,000,000,000,000 years. As you can see, very large quantities of atoms take up very little space which gives us an idea of just how tiny they are. Here is another example: One mole of water with all 6.02 x 10²³ molecules of HO occupies slightly more than a tablespoon.
So how do those tiny atoms come together to make up the stuff in the world around us? Even though atoms are so small, there is a lot of action going on. Each atom is made up of even smaller particles called electrons. The way those electrons place themselves around the atom lead to properties we can experience and observe. In a metal, the tiny atoms are swimming in a sea of electrons which gives them the ability to conduct heat and electricity.
How about water? The electrons in a molecule of water are arranged so that each water molecule is extremely attracted to the one next to it. Because of this they naturally arrange themselves at the atomic level in ways that have big consequences in the world around us. When water freezes, the molecules arrange in a way that creates a lattice that causes ice to float in liquid water. Why is that so important? Because ice floats, a pond or lake will freeze at the top, but below the entire aquatic ecosystem is able to survive. This is an amazing phenomenon of water.
S To Convert Grams To Moles
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
Many chemical calculations require the number of moles of a material, but how do you measure a mole? One common way is to measure the mass in grams and convert to moles. Converting grams to moles is easy with these few steps.
Moles To Grams Conversion Problem
Sometimes you are given moles and need to convert it into grams. This worked example problem shows you how to convert moles to grams.
Problem
Determine the mass in grams of 3.60 mol of H2SO4.
Solution
First, look up the atomic masses for hydrogen, sulfur, and oxygen from the periodic table. The atomic mass is 1.008 for H, 32.06 for S, and 16.00 for O. The formula mass of H2SO4 is:
2 + 32.06 + 4 = 98.08
Thus, one mole of H2SO4 weighs 98.08 grams. This relation provides a conversion factor to go from grams to moles. Using the factor 98.08 g / 1 mol:
grams H2SO4 = 3.60 mol x 98.08 g / 1 mol = 353 g H2SO4
Answer
There are 353 grams of H2SO4 in 3.60 moles of H2SO4.
Recommended Reading: Fsa Algebra 1
How Is A Mole Defined
A mole is defined as 6.02214076 × 1023 of some chemical unit, be it atoms, molecules, ions, or others. The mole is a convenient unit to use because of the great number of atoms, molecules, or others in any substance. The mole was originally defined as the number of atoms in 12 grams of carbon-12, but in 2018 the General Conference on Weights and Measures announced that effective May 20, 2019, the mole would be just 6.02214076 × 1023 of some chemical unit.
Converting Between Mass Number Of Moles And Number Of Atoms
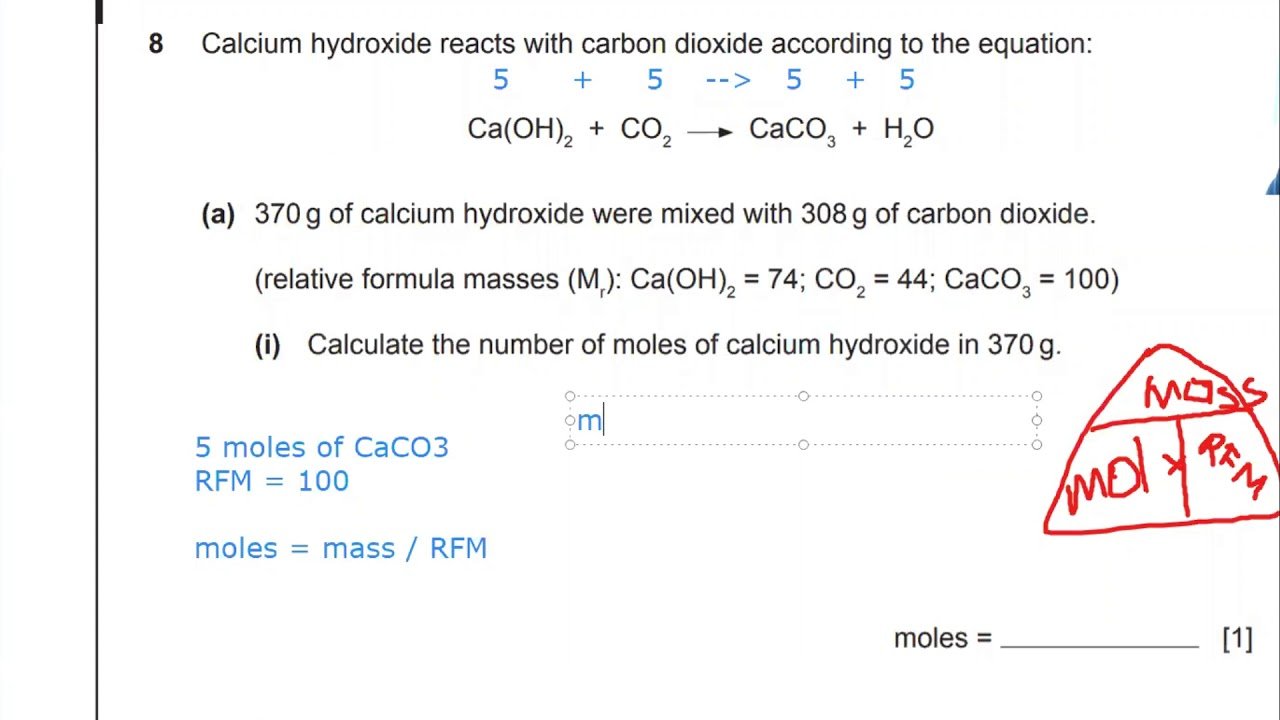
How many moles and how many atoms are contained in 10.0 g of nickel?
According to the periodic table, the atomic mass of nickel is 58.69 amu, which means that the molar mass of nickel is 58.69 g/mol. Therefore, we can divide 10.0 g of Ni by the molar mass of Ni to find the number of moles present.
Using dimensional analysis, it is possible to determine that:
10\text\times \frac}} = 0.170\text
To determine the number of atoms, convert the moles of Ni to atoms using Avogadros number:
0.170\text\times\frac \text}} = 1.02\times10^\text
Given a samples mass and number of moles in that sample, it is also possible to calculate the samples molecular mass by dividing the mass by the number of moles to calculate g/mol.
What is the molar mass of methane if there are 0.623 moles in a 10.0g sample?
\frac_4}_4} = 16.05 \text_4
The molar mass of CH4 is 16.05 g/mol.
Don’t Miss: Algebra Road Trip Project Answer Key
The History Of Avogadros Number:
Though called Avogadros number, the person who originally estimated the number of particles in a certain substance was Josef Loschmidt, who put the value of particles in one cubic centimeter of gas at 2.6867773 x 1025 m-3. The term Avogadros number was coined by a French physicist, Jean Baptiste Perrin. Perrin made an estimate of what he called Avogadros number. Avogadro had been the first physics professor in Italy and had created a hypothesis that suggested gases of equal volume at the same temperature and pressure should contain the same number of particles. Perrin used Loschmidts constant and Avogadros hypothesis to create the Avogadro number.
Constantly Walking The Planck
The road to democratizing measurement involves a quantity from quantum physics called the Planck constant. Conceived by physicist Max Planck in 1900, this constant was introduced at the dawn of quantum mechanics, the theory that describes the behavior of atoms and other things at atomic and subatomic scales too small for us to see.
While the value of the mole is almost unimaginably big, Planck constant is a tiny number, containing 33 zeroes after the decimal point. According to quantum mechanics, an atom only absorbs or doles out energy in package-like chunks or quanta, whose value is equal to a multiple of Plancks constant. Its like a monetary system in which pennies dont exist; you can only buy or sell things in multiples of nickels. The nickel, in this case, is Plancks constant.
How does this relate to mass? Well, this brings in the most famous equation in all of physics: E=mc2. This says that mass can transform into energy, and energy into mass. And one type of energy is equivalent to another type. So, electrical energy can transform completely into mechanical energy, and vice versa. If the electrical energy of a quantum system is proportional to Plancks constant, and it creates a force that balances out the weight of a mechanical system containing a mass, you can measure the amount of that mass using Plancks constant.
In other words, chemistry.
Is this all just theoretical cogitations?
No, it is very real, Vocke says.
Recommended Reading: Segment And Angle Addition Worksheet
Playing The Mass Spec
Avogadro mostly worked with rudimentary instruments: small burners and such. Here in the NIST labs and pretty much everywhere modern chemists work, there are now instruments called mass spectrometers, which are often large boxy things. To Avogadros 19th-century eyes, these might have looked like child-sized coffins or large trunks for sending goods overseas via tall ships.
Mass spectrometers are used to vaporize samples into atoms and molecules and then ionize them . Inside the big box, those ions are accelerated by an electric field and then deflected by a magnetic field where they acquire a trajectory based on their mass- to-charge ratio. Lighter ions are more strongly deflected while heavier ones undergo less deflection. Researchers obtain a spectrum of masses in a sample by observing the different deflections. These mass spectra are like unique fingerprints that can help identify the composition of the original sample. Natural silicon, for example, has three stable isotopic forms and produces a mass spectrum consisting of silicon-28, silicon-29 and silicon-30.
It is easy to get the sense that Rabb really loves to use his mass spectrometer the way someone else might like playing a musical instrument or writing tight and concise code for computers. He likes thinking through the process. He tries to understand how each step will influence the precision of the measurements that result from his work.
Vocke, on the other hand, credits Rabbs chemistry skills.;
Quick Reference Guide For Calculating And Converting Moles:
To convert particles to moles:
Take the number of particles and divide them by Avogadros number.
To convert moles to particles :
Take the number of moles and multiply them by Avogadros number.
To convert grams to moles:
Divide the initial mass of the substance by the compounds molar mass .
To convert moles to mass:
Multiply the starting number of moles with the compounds molar mass.
Also Check: Eoc Fsa Warm Ups Algebra 1 Answers
Converting Moles To Molecules
To convert a number of moles to molecules, you first want to note how many moles are in the substance you have and the chemical makeup of the substance youre analyzing. Going back to the H2O example, lets say you have 4 moles of H2O. Take the number of moles and then multiply it by Avogadros number:
4 mol x 6.022 x 10^23 = 24.0×10^23
You could simplify this number by moving the decimal point one space to the left, to get:
2.4 x 10^24
Note that if you did this youd also need to increase the exponent because it now must reflect the fact that youve shifted the decimal point over.
How Is A Mole Calculated
If you want to know how many moles of a material you have, divide the mass of the material by its molar mass. The molar mass of a substance is the mass in grams of one mole of that substance. This mass is given by the atomic weight of the chemical unit that makes up that substance in atomic mass units . For example, silver has an atomic weight of 107.8682 amu, so one mole of silver has a mass of 107.8682 grams.
mole, also spelled mol, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms, molecules, or other specified particles.
The mole designates an extremely large number of units, 6.02214076 × 1023. The General Conference on Weights and Measures defined the mole as this number for the International System of Units effective from May 20, 2019. The mole was previously defined as the number of atoms determined experimentally to be found in 12 grams of carbon-12. The number of units in a mole also bears the name Avogadros number, or Avogadros constant, in honour of the Italian physicist Amedeo Avogadro . Avogadro proposed that equal volumes of gases under the same conditions contain the same number of molecules, a hypothesis that proved useful in determining atomic and molecular weights and which led to the concept of the mole.
Read Also: Eoc Fsa Warm Ups Algebra 1 Answers
Redefinition Of Si Base Units
In 2011, the 24th meeting of the General Conference on Weights and Measures agreed to a plan for a possible revision of the SI base unit definitions at an undetermined date.
On 16 November 2018, after a meeting of scientists from more than 60 countries at the CGPM in Versailles, France, all SI base units were defined in terms of physical constants. This meant that each SI unit, including the mole, would not be defined in terms of any physical objects but rather they would be defined by constants that are, in their nature, exact.
Such changes officially came into effect on 20 May 2019. Following such changes, “one mole” of a substance was redefined as containing “exactly 6.02214076×1023 elementary entities” of that substance.
Calculating Molarity Given Moles And Volume
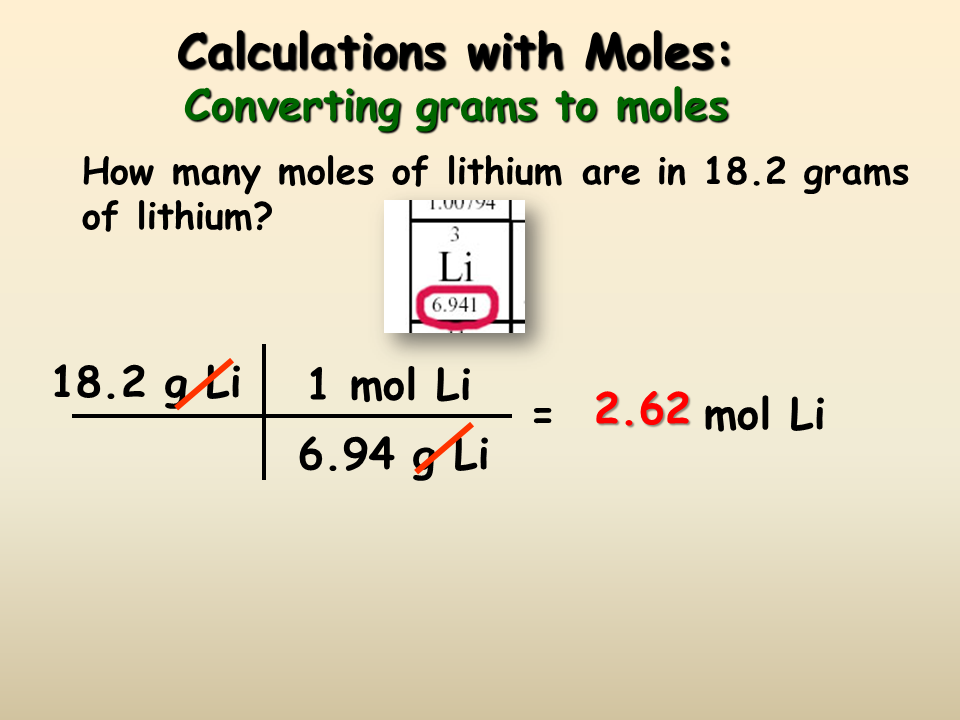
If there are 10.0 grams of NaCl dissolved in water to produce 2.0 L of solution, what is the molarity of this solution?
First, we must convert the mass of NaCl in grams into moles. We do this by dividing by the molecular weight of NaCl .
10.0 \text \times \frac}} = 0.17 \text
Then, we divide the number of moles by the total solution volume to get concentration.
c_i=\frac
c_i=\frac}}
c_i = 0.1 \text
The NaCl solution is a 0.1 M solution.
Don’t Miss: Who Are Paris Jackson’s Biological Parents
Mole A Unit Of Measurement
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
A mole is simply a unit of measurement. In fact, it’s one of the seven base units in the International System of Units . Units are invented when existing units are inadequate. Chemical reactions often take place at levels where using grams wouldn’t make sense, yet using absolute numbers of atoms/molecules/ions would be confusing, too. So, scientists invented the mole to bridge the gap between very small and very large numbers.
Here is a look at what a mole is, why we use moles, and how to convert between moles and grams.
Using Stoichiometry To Calculate Moles
The next step is to inspect the coefficients of each element of the equation. The coefficients can be thought of as the amount of moles used in the reaction. The key is reaction stoichoimetry, which describes the quantitative relationship among the substances as they participate in the chemical reaction. The relationship between two of the reactions participants can be viewed as conversion factors and can be used to facilitate mole-to-mole conversions within the reaction.
Don’t Miss: What Are The Major Specialties In Psychology
How To Calculate Moles From Grams
Chemistry is full of many different confusing conversions. These conversions are important because they ultimately allow us to discover how a particular atom or molecule will interact with other atoms and molecules. Central to chemical conversions is the conversion of grams to moles, and vice versa. A mole is an abstract number that correlates to 6.02 x 10^23 units of a substance present. It doesn’t matter what it is, one mole of it will be 6.02 x 10^23 units. A gram is a scientific measurement of an objects mass. Converting between the two shows us how much a molecule weighs, or how much of it is present.
Converting Moles To Grams
One of the most common chemistry calculations is converting moles of a substance into grams. When you balance equations, you’ll use the mole ratio between reactants and reagents. To do this conversion, all you need is a periodic table or another list of atomic masses.
Example: How many grams of carbon dioxide is 0.2 moles of CO2?
Look up the atomic masses of carbon and oxygen. This is the number of grams per one mole of atoms.
Carbon has 12.01 grams per mole.Oxygen has 16.00 grams per mole.
One molecule of carbon dioxide contains 1 carbon atom and 2 oxygen atoms, so:
number of grams per mole CO2 = 12.01 + number of grams per mole CO2 = 12.01 + 32.00number of grams per mole CO2 = 44.01 gram/mole
Simply multiply this number of grams per mole times the number of moles you have in order to get the final answer:
grams in 0.2 moles of CO2 = 0.2 moles x 44.01 grams/molegrams in 0.2 moles of CO2 = 8.80 grams
It’s good practice to make certain units cancel out to give you the one you need. In this case, the moles canceled out of the calculation, leaving you with grams.
Read Also: Ccl4 Valence Electrons
How To Convert Moles To Molecules With Examples
To convert moles to molecules you will need to use two equations and have at hand Avagadros number and the number of moles in your end substance. See below for examples and formulas.
6.02 x 1023
602,000,000,000,000,000,000,000
In chemistry courses, youll frequently have to convert moles to molecules or molecules to moles using Avogadros number. To do this, youll want to be familiar with the definitions of both moles and molecules, the relationship between the two concepts, and the exact formula which uses Avogadros number.
What Is A Mole
Like all units, a mole has to be defined or else based on something reproducible. The present definition of the mole is defined, but it used to be based on the number of atoms in a sample of the isotope carbon-12.
Today, a mole is Avogadro’s number of particles, which is exactly 6.02214076×1023. For all practical purposes, the mass of one mole of a compound in grams is approximately equal to the mass of one molecule of the compound in daltons.
Originally, a mole was the quantity of anything that has the same number of particles found in 12.000 grams of carbon-12. That number of particles is Avogadro’s Number, which is roughly 6.02×1023. A mole of carbon atoms is 6.02×1023 carbon atoms. A mole of chemistry teachers is 6.02×1023 chemistry teachers. It’s a lot easier to write the word ‘mole’ than to write ‘6.02×1023’ anytime you want to refer to a large number of things. Basically, that’s why this particular unit was invented.
Recommended Reading: Definition Of Span Linear Algebra
Performing Grams And Moles Conversions
Here are some tips for performing these conversions:
- The two problems most commonly encountered are setting up the problem incorrectly, so the units don’t cancel out and give the correct result. It helps to write out the conversion and make sure units cancel. You may want to draw a line through them in complex calculations to keep track of active units.
- Watch your significant figures. Chemistry professors are unforgiving when it comes to reporting an answer, even if you set up the problem correctly.
Moles And The Periodic Table
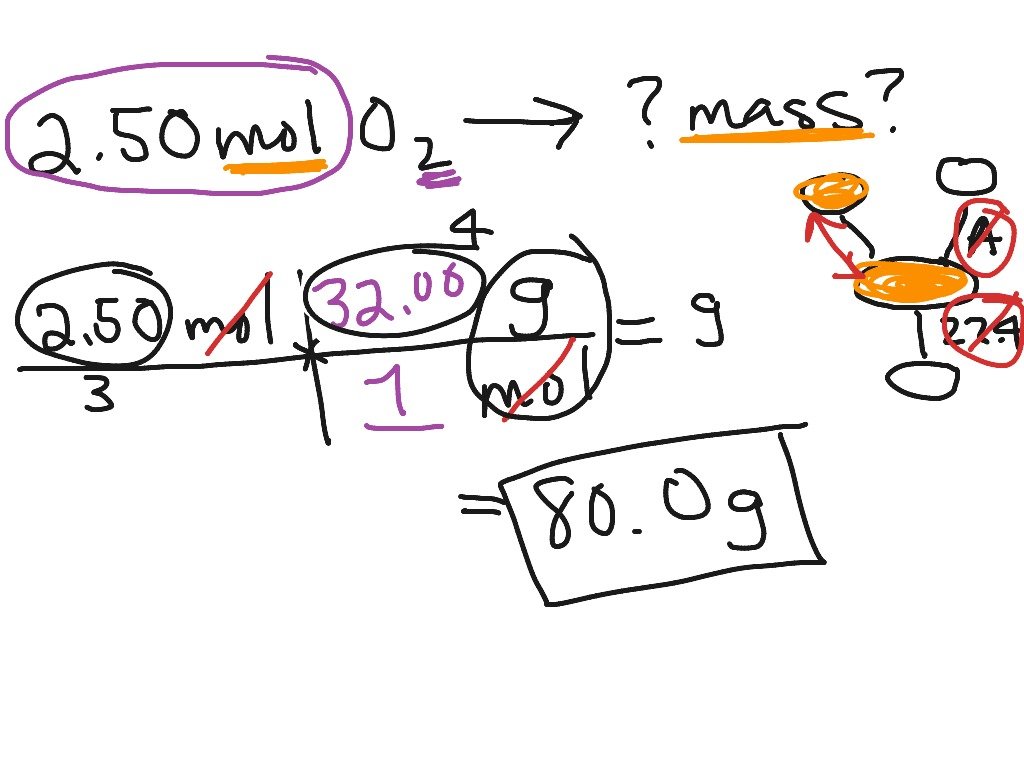
Sometimes a periodic table will simply have the names or abbreviations for each element in the blocks. But sometimes we will see numbers in these blocks. Let’s look at calcium.
We know that the Ca is simply the abbreviation for calcium. But what are those numbers? The ’20’ in the top right corner simply refers to the fact that calcium is element number 20. The 40.08 on the bottom is the important number right now. This is the atomic weight of calcium. The atomic weight of each element is the weight of 1 mole of that particular element.
Also Check: Who Are Paris Jackson’s Biological Parents