Relationship Between The Enantiomers
Enantiomers are a type of stereoisomers in which two molecules are a non-superimposable mirror image of each other.
In other words, one of the enantiomers is a mirror image of the other which cannot be superimposed. In other words, if a mirror looks at one isomer, it would see the other. The two isomers have a different spatial arrangement.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
Metastable And Ground State Notation
The ground state is indicated using the symbol g . The excited states are denoted using the symbols m, n, o, etc. The first metastable state is indicated by the letter m. If a specific isotope has multiple metastable states, the isomers are designated m1, m2, m3, etc. The designation is listed after the mass number .
The symbol sf may be added to indicate isomers capable of spontaneous fission. This symbol is used in the Karlsruhe Nuclide Chart.
Fission Isomers And Shape Isomers
A specific type of nuclear isomer is the fission isomer or shape isomer. Fission isomers are indicated using either a postscript or superscript “f” instead of “m” . The term “shape isomer” refers to the shape of the atomic nucleus. While the atomic nucleus tends to be depicted as a sphere, some nuclei, such as those of most actinides, are prolate spheres . Because of quantum mechanical effects, de-excitation of excited states to the ground state is hindered, so the excited states tend to undergo spontaneous fission or else return to the ground state with a half-life of nanoseconds or microseconds. The protons and neutrons of a shape isomer may be even further from a spherical distribution than the nucleons on the ground state.
Read Also: Lesson 8.1 Practice B Geometry Answers
Origin Of Optical Isomers
To determine whether the compound is optically active or not, we have to first see whether the carbon is attached to four different groups or not. For a better understanding of optical isomerism, let us take an example of two models of organic compound as shown below
These two models have the same bonding arrangement of the atom but a different spatial arrangement. From the above model of A and B, it is clear that the arrangement of the blue and orange group in space is different. Is it possible to align model A exactly like model B by rotating it? The answer is no. The reason for that is if we rotate A the arrangement of other group gets disturbed as shown below
We cannot make the spatial arrangement of A and B exactly the same by rotating them in any direction. A and B is said to be non-superimposable because we cannot make them look exactly.
Let us now see what will happen if a molecule containing two same groups attached to a central carbon atom is rotated as shown in the figure below
Rotating molecule A by 180 degrees will give the same arrangement of the atom as that of B as shown below
From the above explanation, we can conclude that the compound will be optically active only if all the group attached to the central carbon atom are different.
Chiral And Achiral Molecules
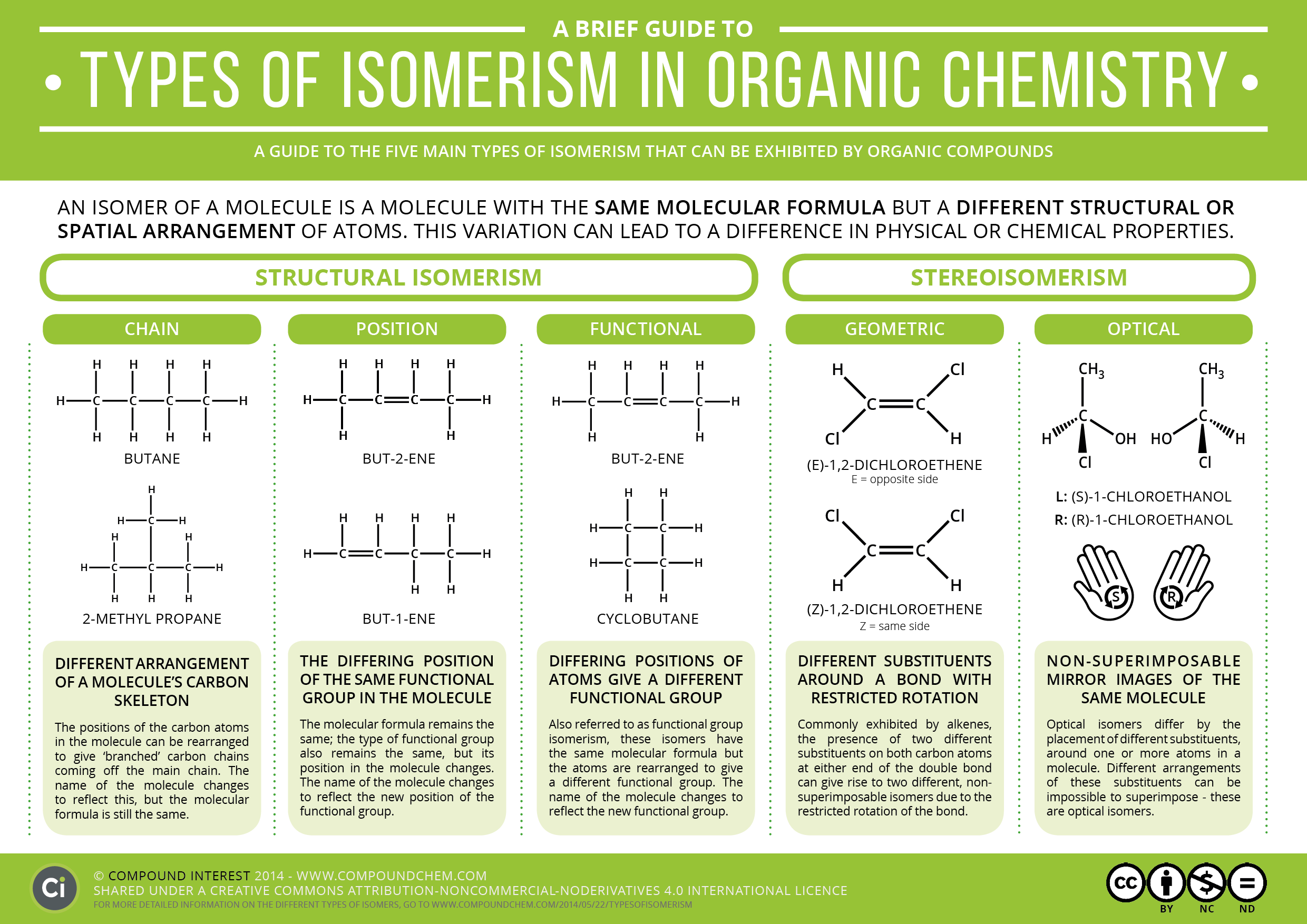
The difference between chiral and achiral molecules can be explained on the basis of the plane of symmetry. If all the attached group to the central carbon atom are different then there is no plane of symmetry. Such a molecule is known as a chiral molecule.
If all the group attached to the central carbon atom are not different then there exist plane of symmetry. Such molecules are called achiral molecules. It is clear that only molecule having chiral centre will show optical isomerism.
Also Check: 10 Branches Of Chemistry
Single Enantiomers Vs Racemic Mixtures
Single enatiomers have less complex and more selective pharmacodyanamic profile as compared to racemic mixture, so have lesser adverse drug reactions, improved therapeutic profile, less chances of drug interactions than racemic mixtures. Single enantiomers seem to be more advantageous over racemic mixtures as – adverse drug reactions occurring due to one enentiomers are avoided, patients are exposed to less amount of drug so body is exposed to the lesser metabolic, renal and hepatic load of drug, there is easier therapeutic drug monitoring of the active pure active enantiomers. A number of drugs are marketed now as single enantiomer like Levosalbutamol, Escitalopram, Naproxen, etc. Many antibiotics have only one enantiomer produced because they are made by fermentation and even the semi-synthetic ones start with the natural fermentation product like quinolones and all penicillins.
What Is Optical Isomerism
Optical isomerism is a case where the isomers display identical characteristics in terms of molecular weight as well as chemical and physical properties. However, they differ in their effect on the rotation of polarized light.
Optical isomerism occurs mainly in substances that have the same molecular and structural formula, but they cannot be superimposed on each other. In simple words, we can say that they are mirror images of each other. Alternatively, it can also be found in substances that have an asymmetric carbon atom.
Typically, optical isomerism is shown by stereoisomers which rotate the plane of polarized light. If the plane of polarized light passing through enantiomer solution rotates in the clockwise direction then the enantiomer is said to exist as form and if the plane of polarized light rotates in anti-clockwise direction then the enantiomer is said to exist in .
Also Read:Isomers, Isomerism, Structural Isomerism
For example, an enantiomer of alanine which rotates the plane of polarized light in clockwise and anti-clockwise direction can be written as alanine and. alanine respectively.
The extent of rotation of plane-polarized light by the two enantiomeric form is exactly the same but the direction of rotation is opposite. Moreover, if the two enantiomer pair are present in equal amount then the resultant mixture is called a racemic mixture. This means that 50% of the mixture exists in form and the other 50% exist in form.
Read Also: Subfields In Psychology Worksheet Answers
Setting Your Browser To Accept Cookies
There are many reasons why a cookie could not be set correctly. Below are the most common reasons:
- You have cookies disabled in your browser. You need to reset your browser to accept cookies or to ask you if you want to accept cookies.
- Your browser asks you whether you want to accept cookies and you declined. To accept cookies from this site, use the Back button and accept the cookie.
- Your browser does not support cookies. Try a different browser if you suspect this.
- The date on your computer is in the past. If your computer’s clock shows a date before 1 Jan 1970, the browser will automatically forget the cookie. To fix this, set the correct time and date on your computer.
- You have installed an application that monitors or blocks cookies from being set. You must disable the application while logging in or check with your system administrator.
What Is Isomerism Exactly
Knowledge of the meanings of the terms molecular formula, structure formula, and configuration is necessary before beginning the study of isomerism. Read this post for information on molecular formula and structure formula.
Before understanding the meaning of the word configuration, it is necessary to know the directions of the bonds made by carbon in different circumstances and the angles formed between them.
Chemistry Notes
le bell and vant hoff in 1874, it was estimated that the four connectivity of carbon are directed from the centre of a rhombus to its four sides and an angle of 109°28 between any two connectors. It has been known in this regard by experiments and the notion of orbital structure
Isomerism: Different types of structural isomerism
1: Position isomerism 2: Ring chain isomerism 3: Metamerism 4: Functional group isomerism 5: Chain isomerism
A) If the four valences of carbon form four single bonds, these four valences of carbon are directed from the centre of a regular tetrahedron to its four vertices. And there is an angle of 109° between any two connectives. In this case the hybridization of carbon is of sp3 type.
B) If two valences of carbon form one double bond and the remaining two valences form two single bonds, then these three bonds of carbon are in the same plane and any two bonds have an angle of 120°. In this case the hybridization of carbon is of sp2 type.
Don’t Miss: What Does Abiotic Mean In Biology
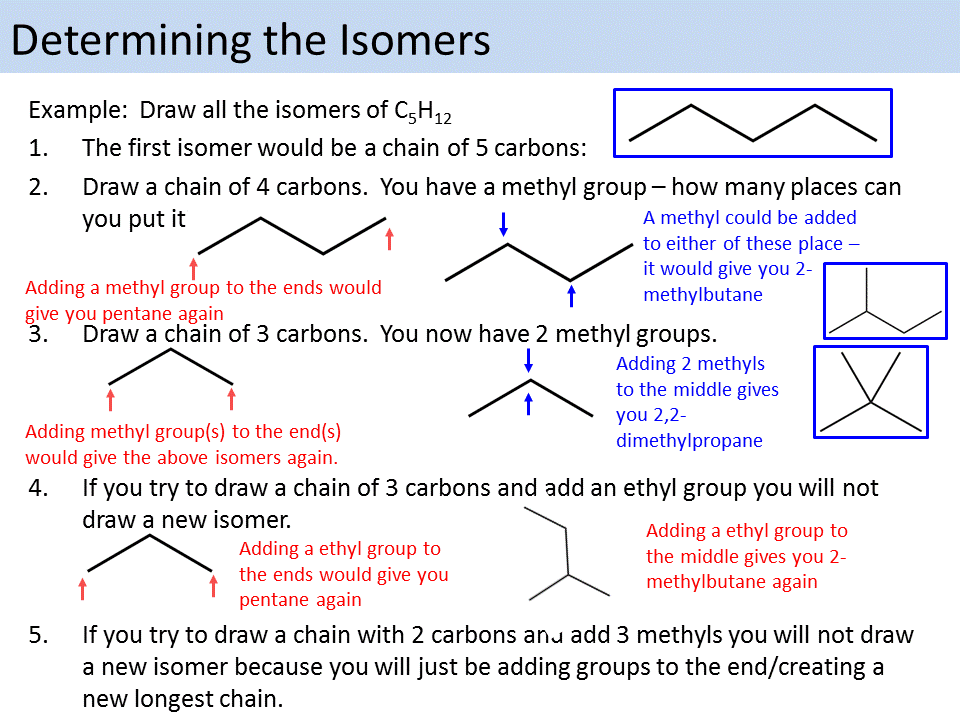
What Is Structural Isomerism
Structural Isomerism, also called compositional isomerism, describes a set of molecules that are made with the same atoms in the same amounts, but are arranged differently.
2-butanol and 1-butanol are structural isomers because they have the same amount and quantity of molecules, but differ in the position of the alcohol group.
Isomerism In Branched Alkanes
In n-alkanes, no carbon is bonded to more than two other carbons, giving rise to a linear chain. When a carbon is bonded to more than two other carbons, a branch is formed. The smallest branched alkane is isobutane. Notice that isobutane has the same molecular formula, C4H10, as n-butane but has a different structural formula. Two different molecules which have the same molecular formula are isomers. Isomers which differ in the connectivity of bonds are constitutional isomers, or structural isomers. Isobutane is a constitutional isomer of n-butane. The prefix “iso” indicates that branches off of the central carbon are equivalent.
n-butane and isobutane are the only constitutional isomers ofC4H10. Pentane, C5H12, has three while hexane, C6H14, has five.
Recommended Reading: Geometry Unit 1 Geometry Basics Answer Key
Optical Isomerism Those Stereoisomers Which Are Mirror Images Of Each Other Or Differ In Optical Activity Are Known As Optical Isomers And This Phenomenon Is Known As Optical Isomerism
Conditions for optical isomerism 1. In optical isomers carbon atoms are attached to four different atoms or groups.
2. Chiral center Optical isomers have chiral centers, it means a carbon atom which is attached to the 4 different groups or atoms.
3. Optical isomer which rotates plane polarized light towards right side is called dextro while the optical isomer which rotates towards left side is called laevo. A mixture containing equal amounts of dextro & laevo isomers is called racemic mixture. Racemic mixtures show no effect on plane polarized light as rotations of d & l cancel out each other.
Optical Isomers example or Example of d & l isomers
D-mannose L-mannose
Those optical isomers which are mirror images of each other are called enantiomers. While which are non-mirror images of each other are called diastereomers.
Geometric Isomerism In Cyclic Compounds
In a cyclic compound the rotation between carbon-carbon single bond is restricted. Thus geometric isomerism is also possible for this type of compounds if two different groups are attached to each carbon. For example, geometrical isomerism is not possible for 1.1-dimethylcyclopropane but it is possible for 1,2-dimethylcyclopropane.
There are two isomers of 1,2-dimethylcyclopropane. One is cis-isomer where two methyl groups are on same side and other is trans-isomer where two methyl groups are on different side.
Also Check: Exponential Growth And Decay Common Core Algebra 1 Homework Answers
Drug Isomerism And Chirality
Stereoisomers differ in pharmacokinetic and pharmacodynamic properties. Pharmacokinetic differences resulting out of stereoisomerism can be in absorption like L-Methotrexate is better absorbed than D-Methotrexate, Esomeprazole is more bioavailable than racemic omeprazole in distribution like S-Warfarin is more extensively bound to albumin than R-Warfarin, hence it has lower volume of distribution. Levocetrizine has smaller volume of distribution than its dextroisomer, d-Propranolol is more extensively bound to proteins than l-Propranolol in metabolism like S-Warfarin is more potent and metabolized by ring oxidation while R-Warfarin is less potent and metabolized by side chain reduction, half life of S-Warfarin is 32 hours while it is 54 hours for R-Warfarin.
Two stereoisomers can compete for binding to same receptors like S-methadone antagonizes respiratory depression action of R-methadone. If the two isomers are of agonist and antagonist type, then racemic mixture acts as partial agonist like picendol and sulfinpyrazone inhibits the metabolism of S-Warfarin significantly but not of R-Warfarin.
Comparison Of Physical Properties Of Two Isomers
Geometric isomers often differ in their physical properties. This is because of the shape of the isomers and the overall dipole moment.
Such as sometime they differ in boiling point. For example the boiling point of cis and trans isomers of 1,2-dichloroethene are 60.3 oC and 47.5 oC.
In cis-isomer, the presence of two dipole bond gives a overall molecular dipole. This results intermolecular dipole-dipole forces. For this force cis isomer have more boiling point than trans-isomer where the two dipole bond is cancelled out because of their position in opposite direction.
Also Check: Unit 1 Test Geometry Basics Answers Key
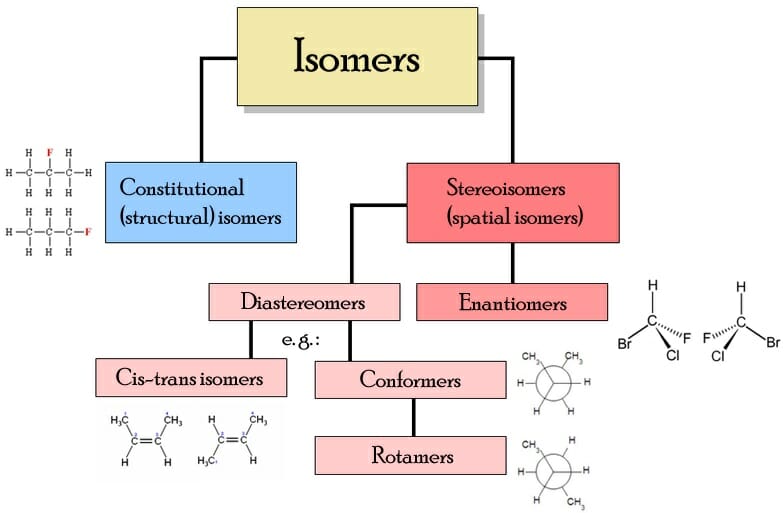
Two Categories Of Isomers Are Structural Isomers And Stereoisomers
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
An isomer is a chemical species with the same number and types of atoms as another chemical species but with distinct properties because the atoms are arranged into different chemical structures. When atoms can assume different configurations, the phenomenon is termed isomerism. There are several categories of isomers, including structural isomers, geometric isomers, optical isomers, and stereoisomers. Isomerization can occur spontaneously or not, depending on whether the bond energy of the configurations is comparable.
What Gets Stored In A Cookie
This site stores nothing other than an automatically generated session ID in the cookie no other information is captured.
In general, only the information that you provide, or the choices you make while visiting a web site, can be stored in a cookie. For example, the site cannot determine your email name unless you choose to type it. Allowing a website to create a cookie does not give that or any other site access to the rest of your computer, and only the site that created the cookie can read it.
Recommended Reading: Lesson 9.5 Geometry Answers
Ionization And Electronic Excitation
The same isomer can also be in different excited states, that differ by the quantum state of their electrons. For example, the oxygen molecule can be in the triplet state or one of two singlet states. These are not considered different isomers, since such molecules usually decay spontaneously to their lowest-energy excitation state in a relatively short time scale.
Likewise, polyatomic ions and molecules that differ only by the addition or removal of electrons, like oxygen O
Isomerization is the process by which one molecule is transformed into another molecule that has exactly the same atoms, but the atoms are rearranged. In some molecules and under some conditions, isomerization occurs spontaneously. Many isomers are equal or roughly equal in bond energy, and so exist in roughly equal amounts, provided that they can interconvert relatively freely, that is the energy barrier between the two isomers is not too high. When the isomerization occurs intramolecularly, it is considered a rearrangement reaction.
An example of an organometallic isomerization is the production of decaphenylferrocene, from its linkage isomer.
- Synthesis of fumaric acid
Industrial synthesis of fumaric acid proceeds via the cis-trans isomerization of maleic acid:
Topoisomerases are enzymes that can cut and reform circular DNA and thus change its topology.
Comparison Of Physical Properties
Cis and trans isomers often have different physical properties. Differences between isomers, in general, arise from the differences in the shape of the molecule or the overall dipole moment.
| cis-2-pentene | |
| Oleic acid | Elaidic acid |
These differences can be very small, as in the case of the boiling point of straight-chain alkenes, such as pent-2-ene, which is 37 °C in the cis isomer and 36 °C in the trans isomer. The differences between cis and trans isomers can be larger if polar bonds are present, as in the 1,2-dichloroethenes. The cis isomer in this case has a boiling point of 60.3 °C, while the trans isomer has a boiling point of 47.5 °C. In the cis isomer the two polar C-Cl bond dipole moments combine to give an overall molecular dipole, so that there are intermolecular dipoledipole forces , which add to the London dispersion forces and raise the boiling point. In the trans isomer on the other hand, this does not occur because the two CCl bond moments cancel and the molecule has a net zero dipole moment .
Thus, trans alkenes, which are less polar and more symmetrical, have lower boiling points and higher melting points, and cis alkenes, which are generally more polar and less symmetrical, have higher boiling points and lower melting points.
As a general trend, trans alkenes tend to have higher melting points and lower solubility in inert solvents, as trans alkenes, in general, are more symmetrical than cis alkenes.
Stability
You May Like: What Does The P Stand For In Pemdas
Solved Examples For You
Q1: What is a chiral carbon?
Ans: If all the four valencies of carbon are satisfied by four different atoms or four different groups of atoms, then carbon is known as chiral carbon.
Q2: What is tautomerism?
Ans: It is the type of isomerism in which two functional isomers exist together in equilibrium. The two forms existing in equilibrium are called as tautomers.
Stereoisomerism Can Be Subdivided Into Following Subtypes
1. Geometrical isomerism It is also known as Cis-Trans isomerism. Those stereoisomers in which isomerism arises due to restricted rotation of carbon-carbon double bond are known as geometrical isomers and this phenomenon is known as geometrical isomerism. In cis-trans isomerism it should be noted that the groups attached to the double bonded carbon atom should be different.
Don’t Miss: What Is An Example Of Movement In Geography