Key Takeaways: How To Calculate Density
- Density is how much matter is contained within a volume. A dense object weighs more than a less dense object that is the same size. An object less dense than water will float on it one with greater density will sink.
- The density equation is density equals mass per unit volume or D = M / V.
- The key to solving for density is to report the proper mass and volume units. If you are asked to give density in different units from the mass and volume, you will need to convert them.
Question 1: What is the density of a cube of sugar weighing 11.2 grams measuring 2 cm on a side?
Step 1:Find the mass and volume of the sugar cube.
Mass = 11.2 gramsVolume = cube with 2 cm sides.
Volume of a cube = 3Volume = 3
Step 2: Plug your variables into the density formula.
density = mass/volumedensity = 1.4 grams/cm3
Answer 1: The sugar cube has a density of 1.4 grams/cm3.
Question 2: A solution of water and salt contains 25 grams of salt in 250 mL of water. What is the density of the salt water?
Step 1: Find the mass and volume of the salt water.
This time, there are two masses. The mass of the salt and the mass of the water are both needed to find the mass of the salt water. The mass of the salt is given, but the only the volume of water is given. We’ve also been given the density of water, so we can calculate the mass of the water.
densitywater= masswater/volumewater
Answer 2: The salt water has a density of 1.1 grams/mL.
Density Of Solid Objects By X
For some crystalline materials, density can be calculated using this equation which is based the molecular mass of the material and on information provided by x-ray crystallography:
- L is Loschmidt or Avogadro’s constant
- a, b, c are the lattice parameters
This equation holds only for crystal systems in which the angles between the crystal axes are ninety degrees, that is, for cubic, tetragonal, and orthorhombic systems. For crystals of lower symmetry the volume of the unit cell is no longer a × b × c, but must be generalized.
As an example of equation , the cubic lattice structure of crystalline sodium chloride is depicted in Figure 1. Each sodium ion is surrounded by six chloride ions and each chloride ion is surrounded by six sodium ions. The sodium chloride molecule, as such, has disappeared. This structural arrangement is in conformity with the x-ray reflection spectra of sodium chloride.
From a study of Figure 1, it can be deduced that each corner chloride ion is common to eight adjacent cubic cells so that only one-eighth of its mass contributes to the mass of the unit cell and thus all eight corner chloride units contribute the mass of one chloride ion. Likewise, the centered chloride ion on each face of the cubic cell is common to two adjacent cubic cells and thus all six of the face-centered chloride ions contribute the mass of three chloride ions. The total mass of the chlorine in the cell is therefore equal to the mass of four chloride ions.
This Example Shows How To Calculate Density
To calculate density of an object, you need to know the mass and volume of the object. These problems show how to calculate density of a solid and liquid.
Another essential concept to bear in mind is whether or not the way to go is sensible. If the object appears heavy because of its size, it ought to have a superior density value. How high? Bear in mind the density water is all about 1 g/cm. Objects less dense than this float in water, while individuals which are more dense sink in water. If the object sinks in water, your density timid player be more than 1!
Density is how much matter is contained within a volume. A dense object weighs more than a less dense object that is the same size. An object less dense than water will float on it one with greater density will sink. The density equation is density equals mass per unit volume or D = M / V. The key to solving for density is to report the proper mass and volume units. If you are asked to give density in different units from the mass and volume, you will need to convert them.
Recommended Reading: Hrw Com Algebra 1
How To Make A Density Column
First make a very simple version using just oil and water.
- Pour some water carefully into a glass or jar.
- Very carefully add about the same volume of cooking oil on top.
- Carefully drop couple of small objects into the mixture and observe what happens. Can you find an object to float on each layer?
What happens if you shake it the jar? You should find that the oil and water mix up and then separate again.
To make a a density column with more layers like the one above, you need lots of different liquids of different densities.
We used: honey, golden syrup , washing up liquid , water and food colouring, vegetable oil in that order.
Density Of Irregularly Shaped Solid Object Heavier Than Water
One of the many statements attributed to the Archimedes principle is that an object immersed in a fluid apparently loses weight by an amount equal to the weight of the fluid displaced. That principle makes it possible to determine of the density of a solid object that is denser than water and is so irregular in shape that its volume cannot be measured directly.
First, the object’s dry weight in air, W
- L is Loschmidt or Avogadro’s constant
- a, b, c are the lattice parameters
This equation holds only for crystal systems in which the angles between the crystal axes are ninety degrees, that is, for cubic, tetragonal, and orthorhombic systems. For crystals of lower symmetry the volume of the unit cell is no longer a × b × c, but must be generalized.
As an example of equation , the cubic lattice structure of crystalline sodium chloride is depicted in Figure 1. Each sodium ion is surrounded by six chloride ions and each chloride ion is surrounded by six sodium ions. The sodium chloride molecule, as such, has disappeared. This structural arrangement is in conformity with the x-ray reflection spectra of sodium chloride.
The molecular mass of sodium chloride is 58.45 g/mol and the cube’s lattice parameters a, b and c are each 0.563 nm. Using Avogadro’s constant of 6.022×1023 and equation above:
- density of NaCl = ÷ = 217.6 × 1023 g/nm3 = 2.176 g/cm3
That is within 0.5 percent of the 2.165 g/cm3 given as the density of NaCl in the Handbook of Chemistry and Physics.
Don’t Miss: Who Are Paris Jackson’s Biological Parents
Density Pressure And Temperature
The density of a material varies with temperature and pressure. This variation is typically small for solids and liquids but much greater for gases. Most materials expand when their temperatures increase. Rising temperatures make the liquid expand in a liquid-in-tube thermometer and bend bimetallic strips. As a result of this expansion, the density of most materials decreases. This effect is caused by a decrease in the atomic number density. This dependence is usually expressed by the coefficient of linear or volume expansion.
Increasing the pressure on an material decreases the volume of the object and thus increases its density via the atomic number density. Compressibility change.
See also: Densest Materials of the Earth
Density Atomic Mass And Atomic Number Density
Since the density of a substance is the total mass of that substance divided by the total volume occupied by that substance, it is obvious, the density of a substance strongly depends on its atomic mass and also on the atomic number density ,
- Atomic Weight. The atomic mass is carried by the atomic nucleus, which occupies only about 10-12 of the total volume of the atom or less, but it contains all the positive charge and at least 99.95% of the total mass of the atom. Therefore it is determined by the mass number .
- Atomic Number Density. The atomic number density , which is associated with atomic radii, is the number of atoms of a given type per unit volume of the material. The atomic number density of a pure material having atomic or molecular weight and the material density is easily computed from the following equation using Avogadros number :
Since nucleons make up most of the mass of ordinary atoms, the density of normal matter tends to be limited by how closely we can pack these nucleons and depends on the internal atomic structure of a substance. The densest material found on earth is the metal osmium, but its density pales by comparison to the densities of exotic astronomical objects such as white dwarf stars and neutron stars.
You May Like: Kw Meaning Chemistry
What Is Charge Density In Chemistry
Charge density analysis for crystal engineering. This review reports on the application of charge density analysis in the field of crystal engineering, which is one of the most growing and productive areas of the entire field of crystallography. While methods to calculate or measure electron density are not discussed in detail, the derived quantities and tools, useful for crystal engineering analyses, are presented and their applications in the recent literature are illustrated. Potential developments and future perspectives are also highlighted and critically discussed.
Chemistry Central Journal 8, 68 . doi. org/10. 1186/s13065-014-0068-xDownload citationReceived: 10 July 2014Accepted: 30 October 2014Published: 16 December 2014DOI: doi. org/10. 1186/s13065-014-0068-xKeywordsCharge density analysisCrystal engineeringSupramolecular chemistryX-ray diffraction.
- Electron densities of studies of organic crystals
- Characterization of Intra- and Inter-molecular interactions
- Co-crystals
- Optical properties from electron density studies
- Endnotes
- Keywords
Video advice: Charge Density 12B
Video advice: Charge density, polarization,polarizability, polarizing power, its variations in the periodic table
What is Charge density, variations of charge density in the periodic table.
Video advice: ELECTRON DENSITY & CHARGE DENSITY
Electron density and charge density in hindi and easy,
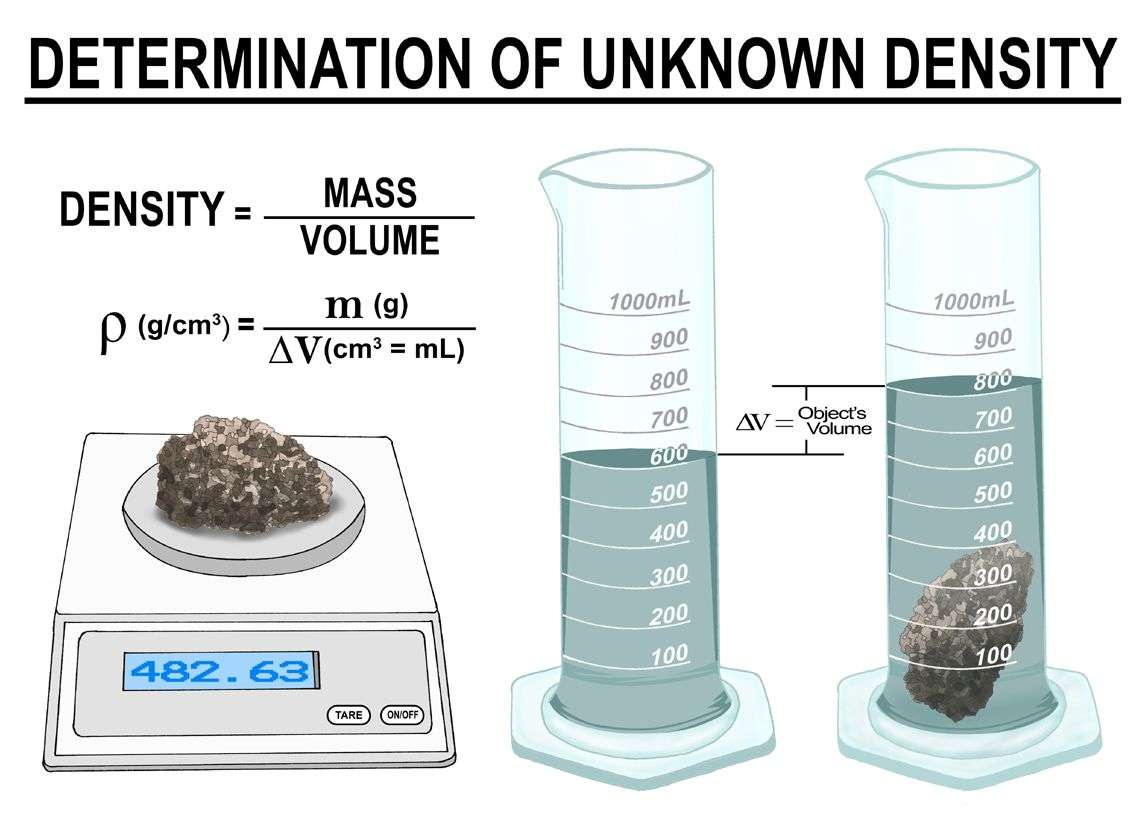
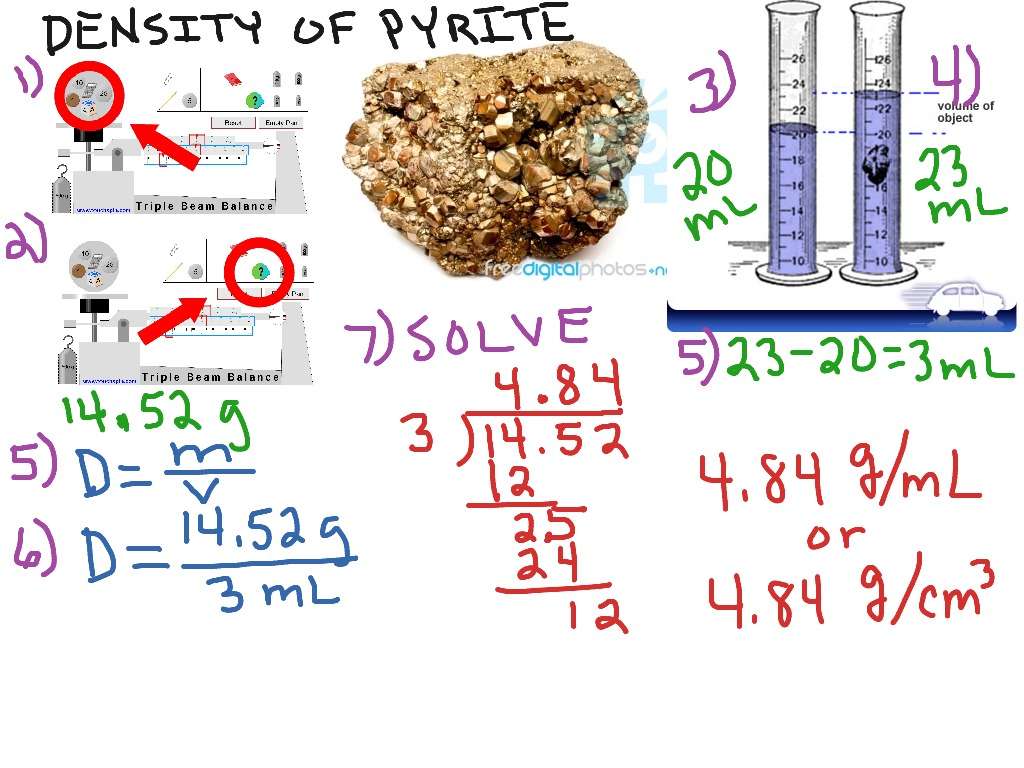
Finding Volume By Displacement
If you’re given a regular solid object, you can measure its dimensions and calculate its volume. Unfortunately, the volume of few objects in the real world can be measured this easily! Sometimes you need to calculate volume by displacement.
How do you measure displacement? Say you have a metal toy soldier. You can tell it is heavy enough to sink in water, but you can’t use a ruler to measure its dimensions. To measure the toy’s volume, fill a graduated cylinder about half way with water. Record the volume. Add the toy. Make sure to displace any air bubbles that may stick to it. Record the new volume measurement. The volume of the toy soldier is the final volume minus the initial volume. You can measure the mass of the toy and then calculate density.
Recommended Reading: Who Coined The Word Geography
Density Of Chemical Elements
Typical densities of various substances are at atmospheric pressure.
Density is defined as the mass per unit volume. It is an intensive property, which is mathematically defined as mass divided by volume:
= m/V
In words, the density of a substance is the total mass of that substance divided by the total volume occupied by that substance. The standard SI unit is kilograms per cubic meter . The Standard English unit is pounds mass per cubic foot .
Finding The Ratio Between Mass And Volume
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
Density is the measurement of the amount of mass per unit of volume. In order to calculate density, you need to know the mass and volume of the item. The formula for density is:
density = mass/volume
The mass is usually the easy part while finding volume can be tricky. Simple shaped objects are usually given in homework problems such as using a cube, brick or sphere. For a simple shape, use a formula to find volume. For irregular shapes, the easiest solution is to measure volume displaced by placing the object in a liquid.
This example problem shows the steps needed to calculate the density of an object and a liquid when given the mass and volume.
Don’t Miss: Cool Math For Kids Happy Wheels
What Is Density In Density Functional Theory
This may be dumb question. Please bear with me.
Density functional theory is a successful theory for electronic structure calculations of materials. In DFT, electron density is the fundamental variable that is proven by the Hohenberg-Kohn theorems. However, I find it difficult to understand how density is used to calculate the energy of the system.
In general, number of electrons per volume will provide the electron density. Okay. Now, I consider Hydrogen atom . If I calculate the electron density, in fact, it depends on how much volume is chosen. If I chose a unit volume, its charge density is 1e. If I double the volume, the charge density halves . What decides this volume? How to chose this volume?
What is density in density functional theory?
The density in density functional theory refers to electron density, which is the object of all DFT calculations. DFT differs from various other wavefunction-based methods which focuses on determining the ground-state $\ce $-dimensional wavefunction*, such as the Hartree-Fock method. Electron density, often denoted as $\rho$ or n, refers to the 3-dimensional function of 3 spatial coordinates describing the distribution of electrons in a given system. It is of interest in DFT as it allows us to determine all observable properties of the system without knowing the exact ground-state wavefunction, hence providing great computational ease. The following is the usual expression of electron density that you would find in resources on DFT:
Density Of Various Materials Examples
1000 kg/m33.98oC . less dense as it freezes ice floats
= m/V = 1/
The specific volume of a substance is the total volume of that substance divided by the total mass of that substance . It has units of cubic meter per kilogram .
heavy water11% greater than water
This difference is caused by the fact, the deuterium nucleus is twice as heavy as hydrogen nucleus. Since about 89% of the molecular weight of water comes from the single oxygen atom rather than the two hydrogen atoms, the weight of a heavy water molecule, is not substantially different from that of a normal water molecule. The molar mass of water is M = 18.02 and the molar mass of heavy water is M = 20.03 , therefore heavy water has a density about 11% greater .
Pure heavy water has its highest density 1110 kg/m3 at temperature 3.98oC . Also heavy water differs from most liquids in that it becomes less dense as it freezes. It has a maximum of density at 3.98 °C , whereas the density of its solid form ice is 1017 kg/m3. It must be noted, the change in density is not linear with temperature, because the volumetric thermal expansion coefficient for water is not constant over the temperature range.
well knowndensity
The density of any substance is the reciprocal of its specific volume .
= m/V = 1/
The specific volume of a substance is the total volume of that substance divided by the total mass of that substance . It has units of cubic meter per kilogram .
Read Also: Glencoe Geometry Concepts And Applications Practice Workbook Answers
Density Of Irregular Shaped Solid Object Heavier Than Water
One of the many statements attributed to the Archimedes principle is that an object immersed in a fluid apparently loses weight by an amount equal to the weight of the fluid displaced. That principle makes it possible to determine of the density of a solid object that is denser than water and is so irregular in shape that its volume cannot be measured directly.
First, the object’s dry weight in air, Wd, is measured. Next, the weight of the object while submerged in water, Ws, is measured. Then the submerged object is removed from the water, the excess surface water is removed and the wet weight in air, Ww, is measured. The density of the object, o, is then obtained from:
and the volumetric porosity fraction, p can be obtained from:
Density Of Various Substances
Perhaps the highest density known is reached in neutron star matter . The singularity at the centre of a black hole, according to general relativity, does not have any volume, so its density is undefined.
| Substance | |
|---|---|
| Aerogel | 3 |
The densest naturally occurring substance on Earth is iridium, at about 22,650 kg/m3. Aerogel is the world’s lightest solid. Also, note the low density of aluminum compared to most other metals. For this reason, aircraft are made of aluminum.
Read Also: Beth Psychopathic Child Now
Food And Drink App: Cooking Temperatures
Because degrees Fahrenheit is the common temperature scale in the United States, kitchen appliances, such as ovens, are calibrated in that scale. A cool oven may be only 150°F, while a cake may be baked at 350°F and a chicken roasted at 400°F. The broil setting on many ovens is 500°F, which is typically the highest temperature setting on a household oven.
People who live at high altitudes, typically 2,000 ft above sea level or higher, are sometimes urged to use slightly different cooking instructions on some products, such as cakes and bread, because water boils at a lower temperature the higher in altitude you go, meaning that foods cook slower. For example, in Cleveland water typically boils at 212°F , but in Denver, the Mile-High City, water boils at about 200°F , which can significantly lengthen cooking times. Good cooks need to be aware of this.
At the other end is pressure cooking. A pressure cooker is a closed vessel that allows steam to build up additional pressure, which increases the temperature at which water boils. A good pressure cooker can get to temperatures as high as 252°F at these temperatures, food cooks much faster than it normally would. Great care must be used with pressure cookers because of the high pressure and high temperature.