Electronic Configuration Of Krypton
Figure 3. the electron configuration of krypton.
How is the size of the orbital related to its energy? Recall that the potential energy of attraction between protons and electrons, which have opposite charges, depends on the distance between them: the closer an electron gets to the protons in the nucleus, the lower its energy will be. Compare the sizes of the 1s and 4s orbitals . Because the 1s orbital is smaller, the average distance of an electron to the nucleus will be smaller than that of the electrons in the 4s orbital. Thats the connection the higher n is, the higher the energy of the orbital.
What about the l in the rule? As mentioned above, l, the angular momentum quantum number, determines the shape of an orbital. In all orbitals for which n > 1, there are areas, called nodes, in which it is extremely unlikely to find an electron. There are two types of nodes: radial and planar . Figure 4 illustrates the radial node in a 2s orbital and a planar node in a 2p orbital . Note that radial node does not cross the nucleus, whereas planar nodes do. s orbitals contain only radial nodes. All other orbitals contain both radial and planar nodes.
General Rules Of Electron Configuration
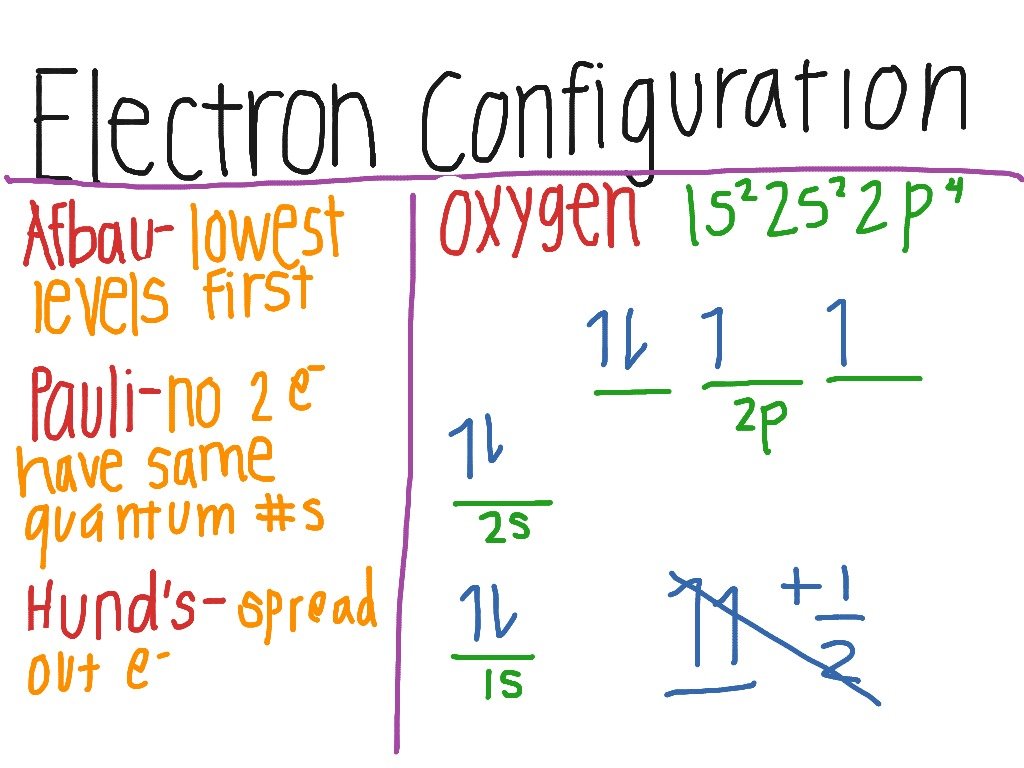
There are a set of general rules that are used to figure out the electron configuration of an atomic species: Aufbau Principle, Hund’s Rule and the Pauli-Exclusion Principle. Before continuing, it’s important to understand that each orbital can be occupied by two electrons of opposite spin . The following table shows the possible number of electrons that can occupy each orbital in a given subshell.
| subshell | |
| 7 , fyz2, fz, fy |
Using our example, iodine, again, we see on the periodic table that its atomic number is 53 . Its complete electron configuration is 1s22s22p63s23p64s23d104p65s24d105p5. If you count up all of these electrons, you will see that it adds up to 53 electrons. Notice that each subshell can only contain the max amount of electrons as indicated in the table above.
Ionization Of The Transition Metals
The naïve application of the aufbau principle leads to a well-known paradox in the basic chemistry of the transition metals. Potassium and calcium appear in the periodic table before the transition metals, and have electron configurations 4s1 and 4s2 respectively, i.e. the 4s-orbital is filled before the 3d-orbital. This is in line with Madelung’s rule, as the 4s-orbital has n+l = 4 while the 3d-orbital has n+l = 5 . After calcium, most neutral atoms in the first series of transition metals have configurations with two 4s electrons, but there are two exceptions. Chromium and copper have electron configurations 3d5 4s1 and 3d10 4s1 respectively, i.e. one electron has passed from the 4s-orbital to a 3d-orbital to generate a half-filled or filled subshell. In this case, the usual explanation is that “half-filled or completely filled subshells are particularly stable arrangements of electrons”. However this is not supported by the facts, as tungsten has a Madelung-following d4s2 configuration and not d5s1, and niobium has an anomalous d4s1 configuration that does not give it a half-filled or completely filled subshell.
Read Also: Broward County Public Schools Algebra 1 Countdown Answers
How Do You Find The Electronic Configuration Of Class 11
The electron configuration of an element describes how electrons are distributed in its atomic orbitals. Electron configurations of atoms follow a standard What is meant by the electronic configuration of an element?What are the three rules that must be followed while writing the electronic configuration of elements?
What Is Electronic Configuration Chemistry Class 9
Electronic configuration is defined as the distribution of electrons into the orbitals of an atom. Every neutral atom consists of a fixed number of electrons which is equal to the number of protons and is called the atomic number.
If you still have questions like the ones below, please contact us for answers:
Subshell
Rules for assigning electrons to orbitals
Magnesium electron configuration
Periodic table of chemical elements
Electrons in outer shell
Also Check: Behaviorism Founder
Electronic Configuration In Periods And Groups
The electronic configuration of an atom is the numerical representation of the arrangement of electrons distributed in the orbitals of the atom. This determines the position of an element in the periodic table and in turn its chemical behavior. It explains how the atoms are held together by the chemical bonds, and the peculiar trends which are observed in the rows and columns of the periodic table. In this article, we will discuss the electronic configuration of elements in the same periods and groups of the periodic table.
Electronic Configuration in Periods
Electronic Configuration in Groups
Elements in the same group have the same number of electrons in their outermost shell leading to similar valence shell electronic configuration. Thus, we observe a similar trend in the properties and chemistry of the elements in the same group.
To learn more about electronic configuration and periodic trends with the vibrant video lectures, download BYJUâS â The Learning App.
Put your understanding of this concept to test by answering a few MCQs. Click âStart Quizâ to begin!
Select the correct answer and click on the âFinishâ buttonCheck your score and answers at the end of the quiz
What Is Called Electronic Configuration
electronic configuration, also called electronic structure, the arrangement of electrons in energy levels around an atomic nucleus. In terms of a more refined, quantum-mechanical model, the KQ shells are subdivided into a set of orbitals , each of which can be occupied by no more than a pair of electrons.
Read Also: Elastic Force Formula
Electron Configuration With Examples
Electron Configuration with Examples
Electrons are not placed at fixed positions in atoms, but we can predict approximate positions of them. These positions are called energy levels or shells of atoms.
- Lowest energy level is 1 and it is denoted with integer n=1, 2, 3, 4, 5, 6 or letters starting from K, L, N to Q. An atom can have maximum 7 energy levels and electrons can change their levels according to their energies.
- Each energy level has different number of electrons. For example, we can find number of electrons in four energy level with following formula 2n2.
1st energy level has
3rd energy level has
2n2=2.32=18 electrons
- Electrons are located energy levels starting from the first energy levels. If one of the energy level is full, then electrons are placed following energy level.
Following pictures show location of electrons of atoms O and Mg.
Number of electrons at the outer shell of atom gives us following classification.
Electron configuration of atom shows, shells, sub shells and number of electrons in sub shells. We examine electron configuration with following examples.
Example: Helium 2
1 is the principal quantum number or energy level
s is the sub-level or sub shell
2 shows the number of electrons in the s sub shell
Example: Chlorine 17
1s22s22p63s23p5
Coefficients 1, 2, 2, 3, and 3 are energy levels of Cl. As you can see p sub shell can have maximum 6 electrons.
Superscripts 2, 2, 6, 2 and 5 are electrons in the sub shells s and p.
Difference Between Conformation And Configuration
September 2, 2018 Posted by Madhu
The key difference between conformation and configuration is that the conformations of the same molecule rapidly interconvert whereas the configurations of the same molecule do not readily interconvert.
Both terms conformation and configuration describes the spatial arrangement of a particular molecule. We use these terms chiefly in organic chemistry in order to determine the spatial arrangement of atoms in organic compounds.
Don’t Miss: Unit 1 Homework 2 Segment Addition Postulate Answer Key
Electronic Configuration Of Sodium
Sodium . The 11 electrons are distributed with 2 electrons in the K shell , 8 electrons in the L shell and 1 electron in the M shell . The M shell in this example is the valence shell. Electronic configurations of all the elements of a period are different , but all the elements of a period have the same number of energy shells in their atoms. For example in the third period the electronic configuration of sodium is 2 , 8, 1 and that of chlorine is 2, 8, 7. Hence energy states are equal in both.
How To Write Electron Configurations
Chemists write electron configurations to describe and communicate the arrangement of electrons around the nucleus of atoms. This notation aids in predicting how atoms will join together to from chemical bonds and their behavior.
What are electron configurations?
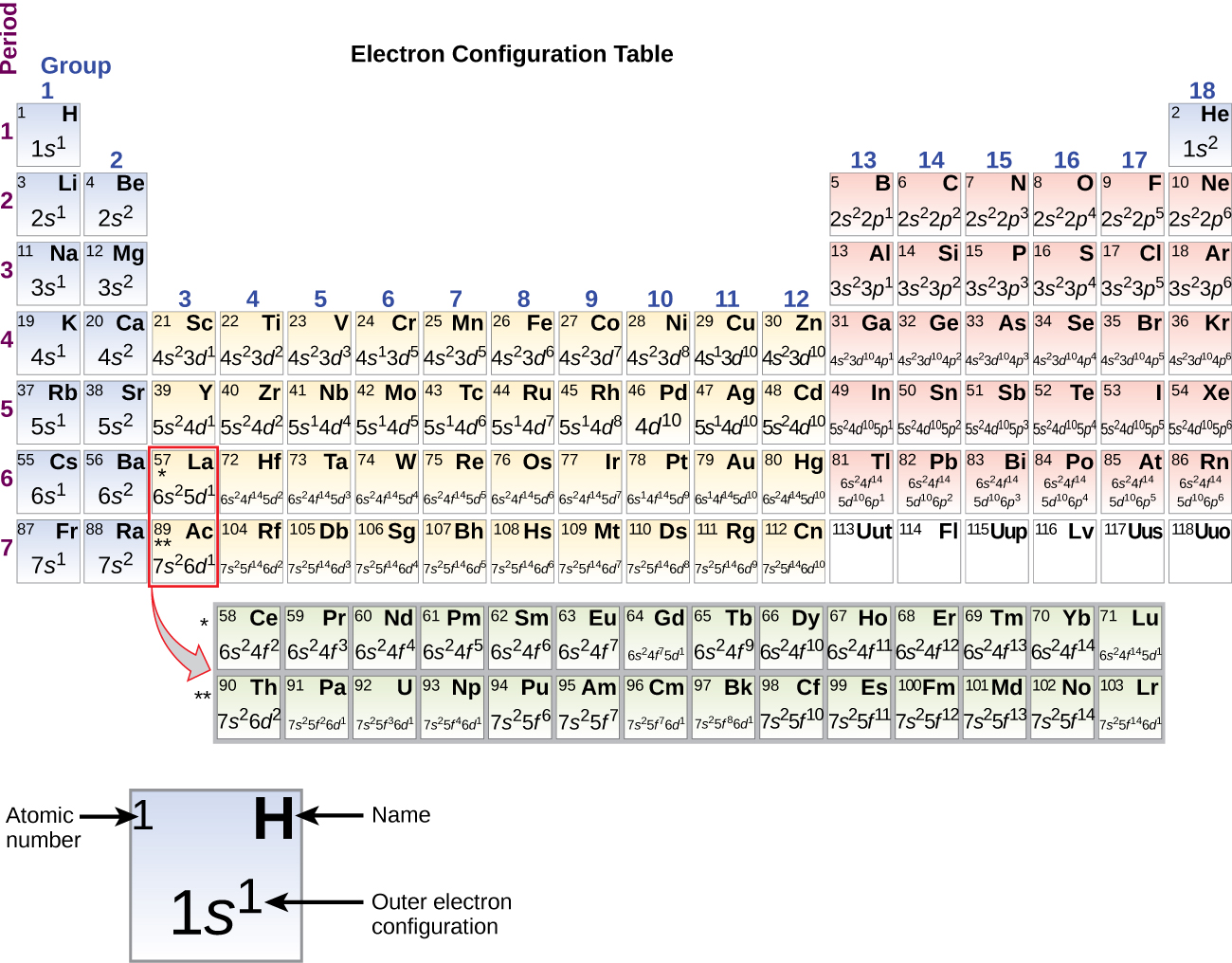
There are two ways to write the electronic configuration of electrons in atoms. The first is more conceptually cohesive and involves using the Periodic Table to write the notation.
Using the Periodic Table to Write Electron Configurations
The second way is more algorithmic and doesn�t really provide a sense of the periodicity and the arrangement of electrons in elements. The level of difficulty is about the same as the Periodic Table method. However this has traditionally the most commonly way of teaching electron configurations.
Using the Configuration Chart to Write Electron Configurations
Also Check: Exponential Growth And Decay Worksheet Algebra 2 Answers
What Is The R And S Configuration And Why We Need It
If we name these two alkyl halides based on the IUPAC nomenclature rules, we get the name as 2-chlorobutanbe for both:
However, they dont look exactly the same as the Cl atom points in different directions wedge and dash. These molecules are not the same compound they are non-superimposable mirror images which are known as enantiomers:
The problem with the wedge and dash notation is that it is not a universal approach and quickly loses validity when we simply look at the molecule from the opposite direction:
So, we need an extra piece of information to distinguish enantiomers by their names properly addressing the stereochemistry as well.
Cahn, Ingold, and Prelog developed a system that, regardless of the direction we are looking at the molecule, will always give the same name .
And that is why this is also known as the Absolute Configuration or most commonly referred to as the R and Ssystem.
Lets see how it works by looking first at the following molecule and we will get back to the 2-chlorobutane after that:
Electronic Configuration Of Iron
Iron is a unique element , which is around and inside us . It has 8 valence electrons and electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d6 , meaning that iron has K shell 2 electrons L shell 8 electrons , M shell 14 and N shell 2 electrons. Under regular conditions iron is a silvery whitre metal, ductile and easily malleable. Iron is a metal with medium activity it forces hydrogen from water solutions of such strong acids as HCl and sulphuric acid forming salts with iron.
You May Like: Who Are Paris Jackson’s Biological Parents
Why Is Electronic Configuration Important
The term electronic configuration has been used since at least the 1920s. Quantum physicist Niels Bohr is credited with developing a model to show how electrons are positioned around a nucleus. Bohrs shell model was used by later scientists, such as Arnold Sommerfeld, to more precisely determine the exact movements and locations of electrons.
When an atom loses or gains electrons, it becomes electrically charged and is referred to as an ion. An ion can also have an electronic configuration. To show this, we simply take the electronic configuration of the neutral atom and add or subtract electrons from the shells.
What Is Electronic Configuration For
The concept of electron configuration establishes the way in which the ordering of electrons within an atom associated with a given element is determined . This allows it to be possible to appreciate at an atomic level everything related to the properties of an element and the mechanism of its chemical reactions .
You May Like: Eoc Fsa Warm Ups Algebra 1 Answers
Summary Conformation Vs Configuration
Conformations and configurations describe the 3D structures of chemical compounds mainly in organic compounds. Even though there are some differences between these two concepts, the key difference between conformation and configuration conformations of the same molecule rapidly interconvert whereas configurations of the same molecule do not readily interconvert.
Reference:
1. Conformations. Master Organic Chemistry RSS. Available here2. Britannica, The Editors of Encyclopaedia. Configuration. Encyclopædia Britannica, Encyclopædia Britannica, Inc., 25 Sept. 2007. Available here
Image Courtesy:
1.Interconversion between eclipsed and gauche conformationsBy Mr.Holmium Own work, via Commons Wikimedia 2.Cis-transBy D.328 2008/03/10 19:38 , via Commons Wikimedia
Introduction: What Are Electron Configurations
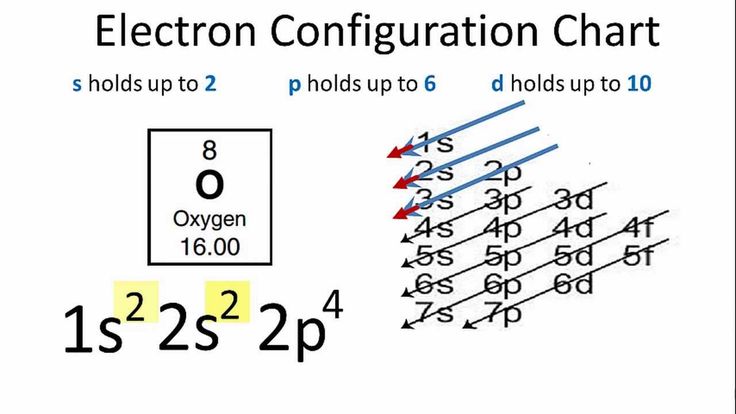
The electron configuration of an element describes how electrons are distributed in its atomic orbitals. Electron configurations of atoms follow a standard notation in which all electron-containing atomic subshells are placed in a sequence. For example, the electron configuration of sodium is 1s22s22p63s1.
However, the standard notation often yields lengthy electron configurations . In such cases, an abbreviated or condensed notation may be used instead of the standard notation. In the abbreviated notation, the sequence of completely filled subshells that correspond to the electronic configuration of a noble gas is replaced with the symbol of that noble gas in square brackets. Therefore, the abbreviated electron configuration of sodium is 3s1 .
Electron Configurations are useful for:
- Determining the valency of an element.
- Predicting the properties of a group of elements .
- Interpreting atomic spectra.
This notation for the distribution of electrons in the atomic orbitals of atoms came into practice shortly after the Bohr model of the atom was presented by Ernest Rutherford and Niels Bohr in the year 1913.
Also Check: Segment Addition Worksheet Answer Key
Energy Of Ground State And Excited States
The energy associated to an electron is that of its orbital. The energy of a configuration is often approximated as the sum of the energy of each electron, neglecting the electron-electron interactions. The configuration that corresponds to the lowest electronic energy is called the ground state. Any other configuration is an excited state.
As an example, the ground state configuration of the sodium atom is 1s2 2s2 2p6 3s1, as deduced from the Aufbau principle . The first excited state is obtained by promoting a 3s electron to the 3p orbital, to obtain the1s2 2s2 2p6 3p1 configuration, abbreviated as the 3p level. Atoms can move from one configuration to another by absorbing or emitting energy. In a sodium-vapor lamp for example, sodium atoms are excited to the 3p level by an electrical discharge, and return to the ground state by emitting yellow light of wavelength 589 nm.
Usually, the excitation of valence electrons involves energies corresponding to of visible or ultraviolet light. The excitation of core electrons is possible, but requires much higher energies, generally corresponding to x-ray photons. This would be the case for example to excite a 2p electron of sodium to the 3s level and form the excited 1s2 2s2 2p5 3s2 configuration.
The remainder of this article deals only with the ground-state configuration, often referred to as “the” configuration of an atom or molecule.
Corrosionpedia Explains Electron Configuration
The electron configuration of an atom describes the orbitals occupied by electrons on the atom. The basis of this prediction is a rule known as the Aufbau principle, which assumes that electrons are added to an atom, one at a time, starting with the lowest energy orbital, until all of the electrons have been placed in an appropriate orbital.
The electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or anion by compensating with the loss of or gain of electrons in their subsequent orbitals. Many of the physical and chemical properties of elements can be correlated to their unique electron configurations.
The most widespread application of electron configurations is in the rationalization of chemical properties, in both inorganic and organic chemistry. In effect, electron configurations, along with some simplified form of molecular orbital theory, have become the modern equivalent of the valence concept, describing the number and type of chemical bonds that an atom can be expected to form. A fundamental application of electron configurations is in the interpretation of atomic spectra.
Don’t Miss: Geometry Segment And Angle Addition Worksheet Answers
What Is Conformation
Conformation refers to different arrangements of atoms in a molecule that can readily interconvert. These interconversions can occur at room temperature easily. Therefore, these structures are highly flexible.
Figure 01: Interconversion of two Conformations via Rotation
However, the different structures cannot be separated from each other. These different structures result from rotations around carbon to carbon single bonds . Anyway, we can get different conformations for the same molecule.
How Can You Use This
Lets summarize what weve discussed so far:
Up to Z = 20 , the rule correctly predicts:
- Orbital energy levels
The order of occupancy of the orbitals
- The physical meaning of the rule is related to the size and shape of a given orbital.
For Z > 20 :
- The rule is not able to correctly predict orbital energy levels.
- Even when we know the orbital energies, this knowledge is not sufficient to predict the order of filling. Other factors, such as d vs. s electron repulsions must be considered. .
- Although its physical meaning is no long sufficient, the rule still correctly predicts the order of filling. Except where it doesnt and we invoke exceptions.
The first point to be taken from this is that the rule is a model and that it works until it doesnt. If you choose to teach it as a model and connect it to some of the physical meaning discussed above, its a great example of how models can be both useful and also fail.
The story outlined above has the potential to be much more fulfilling for your students than Memorize the diagram, learn to use it, and youre guaranteed to get the right answer. But its a tough story to tell by just waving your hands. You need a model to tell it, and the model needs the following features:
References:
Recommended Reading: Ccl4 Valence Electrons