What Is Alkaline In Human Body
Alkalinity means that something has a pH higher than 7. The human body is naturally slightly alkaline , with a blood pH of around 7.4. The stomach is acidic, which allows it to digest food. The pH of saliva and urine changes depending on diet, metabolism, and other factors.
What does alkali feel like?
Alkalis feel soapy when they get on your skin, so it is easy to tell when you have had an accident and must wash your hands. Just like concentrated acids, concentrated alkalis are corrosive. They can attack metals and destroy skin if spilled, so their containers are labelled with a warning symbol.
What Is An Element In Chemistry
Chemical elements. Atomic elements. Simple substances. Elements of the periodic table. These are all different terms for the same concept. A long time ago, elements used to mean earth, air, wind and fire but that is no longer true. The idea of an element, a basic building block of matter, has piqued the curiosity of mankind for many ages. But in chemistry what exactly is an element how do we define it?
What Is An Alkali In Chemistry
An alkali is a substance that produces hydroxide ions, OH -, when dissolved in water. Strong acids completely ionise in water. They break up completely to produce a high concentration of hydrogen ions in the solution.
What is alkali in one word answer?
The definition of an alkali is a soluble salt that comes from the ashes of plants and is made up of mostly potassium or sodium carbonate. A carbonate or hydroxide of an alkali metal, the aqueous solution of which is bitter, slippery, caustic, and characteristically basic in reactions.
Don’t Miss: Why Is Physics So Hard
Types Of Compound Bonds
Compounds are often categorized by the type of bond that holds them together. There are two primary types of bonds:
- Covalent bonds. Bonding occurs when two nonmetal atoms share pairs of electrons to form a stable bond. Examples of covalent compounds include water, carbon dioxide and hydrogen chloride .
- Ionic bonds. Bonding occurs when a metal atom and a nonmetal atom exchange valence electrons to form a stable bond. Examples of ionic compounds include sodium chloride , magnesium sulfate and sodium bicarbonate .
Not all compounds fit neatly into one category or the other. Some compounds, such as potassium Sulfate and sodium hydroxide, contain both covalent and ionic bonds.
Compounds are sometimes distinguished by whether they’re made up of molecules. A molecule is a structure that consists of two or more atoms that are chemically bonded together. The structure might be an elemental or compound molecule. An elemental molecule contains only one type of atom, such as ozone or chlorine . A compound molecule is made up of one or more different elements. Covalent compounds are built on these types of molecules and are often referred to as molecular compounds.
A small subset of elements — helium, neon, argon, krypton, xenon and radon — are known as noble or inert gases and don’t readily bond with other elements to form compounds. However, elements such as oxygen, chlorine and fluorine readily combine with other elements to form compounds.
Definition Of An Element
We can define an element, as a type of matter that can not be broken down into a simpler substance by chemical means. The only way to break down an element, or change it into a different element, is via a nuclear reaction. You can crush an element, throw it, burn it, stomp on it, or dissolve it in acid but you still wont transform, decompose or change the substance into another element. But you might make a mess.
It is important to note, that an element can take many different forms. Lets talk about an example sodium. Pure sodium is a shiny, highly reactive metal. Sodium is also found in table salt, in the form of sodium ions, which are bonded to chlorine atoms. These sodium ions are not shiny or reactive like elemental sodium, but table salt still contains the element sodium. And if we dissolve salt in water, the sodium ions are dissolved in solution. The solution contains the element sodium, but it is not in elemental form. The elemental form of an element is when it exists not chemically combined with any other atom or ion.
Recommended Reading: What Is Input And Output In Math
What Are 5 Reasons Why Chemistry Is Important
Here are some of the best reasons to study chemistry.
- Chemistry helps you to understand the world around you.
- Basic knowledge of chemistry helps you to read and understand product labels.
- Chemistry can help you make informed decisions.
- Chemistry is at the heart of cooking.
- A command of chemistry can help keep you safe!
What Is An Acid And A Base Organic Chemistry
Here, acids are defined as being able to donate protons in the form of hydrogen ions whereas bases are defined as being able to accept protons. This took the Arrhenius definition one step further as water is no longer required to be present in the solution for acid and base reactions to occur.
What are bases in organic chemistry?
An organic base is an organic compound which acts as a base. Organic bases are usually, but not always, proton acceptors. For example, amines or nitrogen-containing heterocyclic compounds have a lone pair of electrons on the nitrogen atom and can thus act as proton acceptors.
Which is the strongest acid organic chemistry?
Conversely, ethanol is the strongest acid, and ethane the weakest acid.
You May Like: Rational Equations Worksheet Algebra 2
What Is An Alkali Simple Definition
alkali, any of the soluble hydroxides of the alkali metalsi.e., lithium, sodium, potassium, rubidium, and cesium. Alkalies are strong bases that turn litmus paper from red to blue they react with acids to yield neutral salts and they are caustic and in concentrated form are corrosive to organic tissues.
How Important Is Chemistry In A Relationship
Having chemistry is a precursor to building lasting commitment and trust in a relationship. Chemistry keeps the relationship interesting over time, as the emotional closeness will remain when two people truly have chemistry. Relationship chemistry means that deep conversation and comfortability will come naturally.
Also Check: What Is Basic Research In Psychology
Chemistry Is Everywhere: In The Morning
Most people have a morning ritual, a process that they go through every morning to get ready for the day. Chemistry appears in many of these activities.
These are just a few examples of how chemistry impacts your everyday life. And we havent even made it to lunch yet!
View the video The Chemical World by Dr. Jessie A. Key for an introduction to the science of chemistry and how it fits into our everyday lives.
Key Takeaways
- Chemistry is the study of matter and its interactions with other matter and energy.
- Matter is anything that has mass and takes up space.
- Matter can be described in terms of physical properties and chemical properties.
- Physical properties and chemical properties of matter can change.
- Matter is composed of elements and compounds.
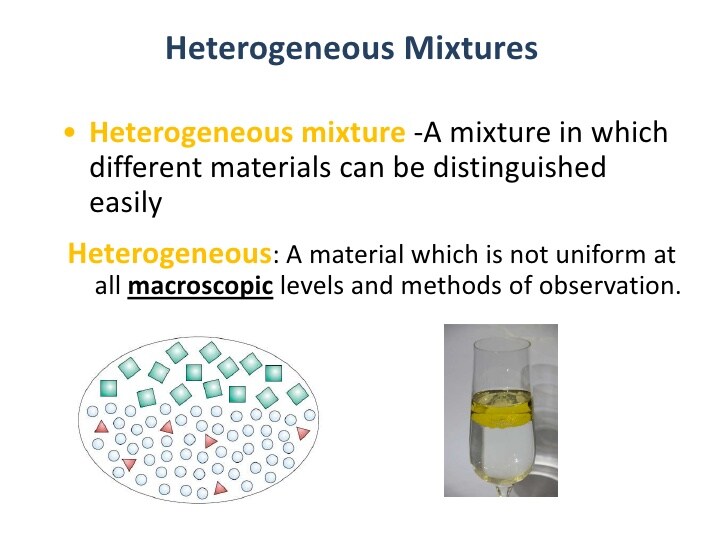
- Combinations of different substances are called mixtures.
- Elements can be described as metals, nonmetals, and semimetals.
Exercises
Chemical Elements And Compounds
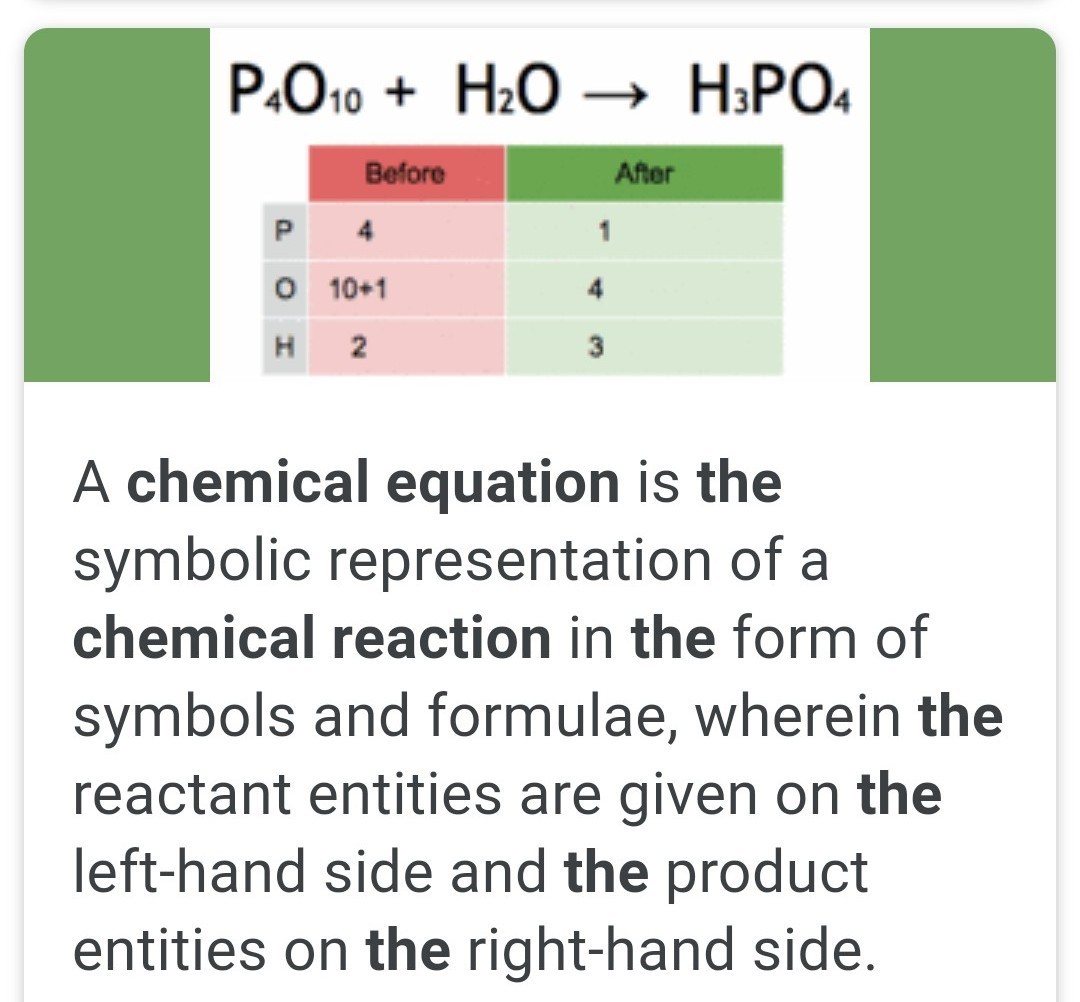
A chemical substance made up of only one atom is called an element. There are 118 known elements, but only 98 of them are found naturally on Earth. Each of these elements is represented by a chemical symbol and can be found in theperiodic table of elements.
A chemical compound is a pure substance that is made up of two elements that are chemically combined. Compounds are chemicals made up of more than one kind of atom. Water is an example of a chemical compound of hydrogen and oxygen. This means that every particle or molecule of water has hydrogen and oxygen atoms that are bonded together.
Elements and compounds are often found in mixtures. A mixture is a combination of two or more substances where each substance has its own identity. In general, these combinations can be separated into their component substances. An example of a mixture is soil and air.
Don’t Miss: Geometry Unit 6 Test Answer Key
What Is Gravimetry And Titrimetry
The key difference between gravimetric and titrimetric analysis is that gravimetric analysis measures the quantity of an analyte using weight, whereas titrimetric analysis measures the quantity of an analyte using volume. If we measure volume, we call it a volumetric analysis or a titrimetric analysis.
What Does Pink Phenolphthalein Mean
Phenolphthalein, an acid-base indicator used to test the pH of a solution, turns pink due to the presence of a weak base. Although the anions are pink, the solution remains colorless in the presence of an acid. If the pH of the solution is 8.2 or above, the number of anions increases, causing the solution to turn pink.
Read Also: Holt Algebra 1 Chapter 2 Test Answers
Easy Definition Of Chemistry
Chemistry, at its most basic, is the branch of science which studies the nature of, and changes in, physical matter that is, chemicals. Whereas physics studies the composition of matter from the perspective of movement and energy, chemistry does so from the perspective of analyzing how matter is structured through atoms and molecules as well as how those structures undergo change through chemical processes called reactions. The majority of chemists are currently involved in the study of the so-called organic compounds molecules based on the complex bonding capabilities of carbon atoms. In the past, however, most interest lay in inorganic compounds, common examples of which include water and table salt.
Fred Senese of Frostburg State University identifies five basic components which must go into a definition of chemistry: chemistry is a branch of science as a science, it is a systematic study of natural phenomena, using the scientific method and rigorous testing protocols to build viable theories it is the study of the composition and properties of matter specifically, it studies chemical reactions within that matter and it is divided into organic and inorganic chemistry.
Messy Definitions
Instead of muddling further through the details of Russells and Seneses work, it might now be best to turn to the two most basic parts of an easy definition of chemistry.
1. Chemistry Studies Matter
2. Chemistry is Science
What Is Z And A In Chemistry
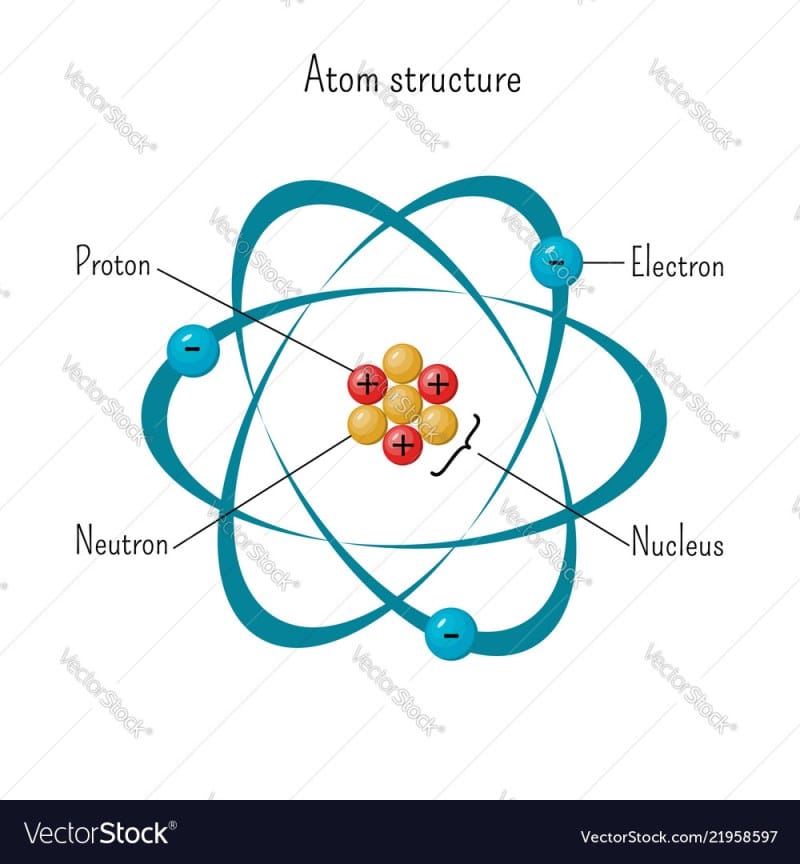
The number of protons and neutrons combines to give us the mass number of an atom. It is represented using the letter A. The atomic number of an atom is equal to the number of protons in the nucleus of an atom or the number of electrons in an electrically neutral atom. It is represented using the letter Z.
Read Also: What Is The Period In Math
Chemistry As A Physical Science
Chemistry is typically considered a physical science, as defined by the Encyclopedia Britannica , because the study of chemistry does not involve living things. Most of the chemistry involved in research and development, such as making new products and materials for customers, falls within this purview.
But the distinction as a physical science becomes a bit blurry in the case of biochemistry, which explores the chemistry of living things, according to the Biochemical Society . The chemicals and chemical processes studied by biochemists are not technically considered “living,” but understanding them is important to understanding how life works.
What Do Chemists Do
Chemists work in a variety of fields, including research and development, quality control, manufacturing, environmental protection, consulting and law. They can work at universities, for the government or in private industry, according to the ACS .
Here are some examples of what chemists do:
Research and development
In academia, chemists performing research aim to further knowledge about a particular topic, and may not necessarily have a specific application in mind. Their results, however, can still be applied to relevant products and applications.
In industry, chemists in research and development use scientific knowledge to develop or improve a specific product or process. For example, food chemists improve the quality, safety, storage and taste of food pharmaceutical chemists develop and analyze the quality of drugs and other medical formulations and agricultural chemists develop fertilizers, insecticides and herbicides necessary for large-scale crop production.
Sometimes, research and development may not involve bettering the product itself, but rather the manufacturing process involved in making that product. Chemical engineers and process engineers devise new ways to make the manufacturing of their products easier and more cost effective, such as increasing the speed and/or yield of a product for a given budget.
Environmental protection
Also Check: What Is The Importance Of Biology In Our Daily Life
How Elements Came To Be Defined Correctly
In 1913, chemistry and physics were topsy-turvy. Some big hitters – including Dmitri Mendeleev – were talking seriously about elements lighter than hydrogen and elements between hydrogen and helium. Visualizing the atom was a free-for-all, and Mendeleev’s justification for a periodic table based on the elements’ atomic weights was falling apart at the seams.
This is the story of how Henry Moseley brought light to the darkness.
The Most Abundant Elements
With only one proton, hydrogen is the simplest, lightest element, followed by helium, which has two protons. Oxygen atoms have eight protons.
At 75 percent, hydrogen is the most abundant element in the universe, followed by helium at 23 percent, then oxygen at 1 percent. All of the other elements make up the remaining 1 percent.
In the earths crust, oxygen is the most abundant element, followed by silicon and aluminum .
Element Names and NumbersAll of the elements have been named. Some of these names are familiar to us, such as nitrogen and sodium, and some are less familiar, such as dysprosium and roentgenium.
We can also name elements using their atomic numbers. For example, element 1 is hydrogen, element 2 is helium, element 3 is lithium, element 8 is oxygen, etc.
How Many Elements Are There?There are currently 118 accepted elements.
We use the periodic table to display all of the elements in an organized way.
Elements Ancient and ModernSome elements have been known for thousands of years, and we do not know who discovered them. These are: antimony, arsenic, carbon, copper, iron, gold, lead, mercury, silver, sulfur, and tin.
All other elements have been discovered since 1669: this was the year Hennig Brand became the first known person to discover a new element – phosphorus.
Search the Dictionary for More Terms
Recommended Reading: What Is Ph In Biology
Chemicals In Our Daily Lives
Chemical compounds are found everywhere, from the food we eat to the air we breathe. Artificial chemicals in food help it to remain fresh and preserve its flavour. Some chemical compounds are even used to enhance food textures, making them crunchy, chewy, or smooth. However, not all chemical compounds can be good for us. MSG is a chemical compound that is added to food to improve the flavour. Using MSG can have negative effects on our health which may cause headaches and other negative reactions.
Disclaimer
All content published on the ReAgent.co.uk blog is for information only. The blog, its authors, and affiliates cannot be held responsible for any accident, injury or damage caused in part or directly from using the information provided. Additionally, we do not recommend using any chemical without reading the Material Safety Data Sheet , which can be obtained from the manufacturer. You should also follow any safety advice and precautions listed on the product label. If you have health and safety related questions, visit HSE.gov.uk.
What Are The Steps Of Titration
Terms in this set
Recommended Reading: Who Is Paris Jackson’s Biological Father
Why Are Titrations Done In Chemistry
A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Knowing the volume of titrant added allows the determination of the concentration of the unknown. Often, an indicator is used to usually signal the end of the reaction, the endpoint.
Can A Coefficient Be Negative
Coefficients are numbers that are multiplied by variables. Negative coefficients are simply coefficients that are negative numbers. An example of a negative coefficient would be -8 in the term -8z or -11 in the term -11xy. The number being multiplied by the variables is negative.
What is 9th coefficient?
Coefficient of Polynomial: Each term of a polynomial has a coefficient. So, in p=9×3 3×2 +8x 2, the coefficient of x3 is 9, the coefficient of x2 is -3, the coefficient of x is 8 and 2 is the coefficient of x0. are examples of constant polynomials. The constant polynomial 0 is called the zero polynomial.
Which is the best definition of coefficient?
The definition of a coefficient is a number or symbol that helps to provide a mathematical result. A number, constant for a given substance, used as a multiplier in measuring the change in some property of the substance under given conditions. The coefficient of expansion.
You May Like: What Is Lithology In Geography