What Is The Ph Of A Cell
Hydrogen ions play a central role in the lives of cells. For example, changes in hydrogen ion concentration are intimately tied to the charge of side chains in proteins. This charge state, in turn, affects the activity of enzymes as well as their folding and even localization. Further, the famed ATP synthases that churn out the ATPs that power many cellular processes are driven by gradients in hydrogen ions across membranes.
The abundance of these ions and, as a result, the charge state of many compounds is encapsulated in the pH defined as
pH=-log10
where denotes the concentration or more formally the activity of the charged hydrogen ions . We are careful to divide the hydrogen ion concentration by a so-called standard state concentration, the agreed upon value is 1M, in order to ensure that when taking the log we have a unitless quantity. This step is often skipped in textbooks.
The integer 7 is often etched in our memory from school as the pH of water, but there is nothing special about the integral value of 7. Water has a neutral pH of about 7, with the exact value varying with temperature, ionic strength and pressure. What is the pH inside the cell? Just like with other parameters describing the state of molecules and cells, the answer depends on physiological conditions and which compartment within the cell we are considering . Despite these provisos, crude generalizations about the pH can be a useful guide to our thinking.
In Summary: Buffers Ph Acids And Bases
The pH of a solution is a measure of the concentration of hydrogen ions in the solution. A solution with a high number of hydrogen ions is acidic and has a low pH value. A solution with a high number of hydroxide ions is basic and has a high pH value. The pH scale ranges from 0 to 14, with a pH of 7 being neutral. Buffers are solutions that moderate pH changes when an acid or base is added to the buffer system. Buffers are important in biological systems because of their ability to maintain constant pH conditions.
What Is Biological Importance Of Ph
Cells work best at very specific pH’s, too low or high and they will not function properly.
All living things are water-based systems, which means that they depend heavily on aqueous equilibria, especially acid-base equilibria. Therefore, all the acid-base and pH concepts we have discussed so far are extremely important to biochemistry, which is the study of the chemistry of biological systems.
Just as in other acid-base systems, biological macromolecules act as acids and bases by donating and accepting protons. However, due to the size of these molecules, they often contain several different groups that accept or donate protons instead of just one such group. Thus, we talk about macromolecules as having acidic and basic groups rather than as being acids and bases. These acidic and basic groups act as weak acids and bases, with Ka values which determine the extent of dissociation of the group depending on the pH of the system. Therefore, changes in the pH around the macromolecule will determine which groups are protonated and which are not, which in turn determines properties of the molecule. This is especially important for enzymes, which are proteins that act as catalysts for important biological reactions. Most enzymes only work within a certain pH range.
You May Like: Different Fields Of Chemistry
Variation Of Ph Across The United States
The pH of precipitation, and water bodies, vary widely across the United States. Natural and human processes determine the pH of water. The National Atmospheric Deposition Program has developed maps showing pH patterns, such as the one below showing the spatial pattern of the pH of precipitation at field sites for 2002. You should be aware that this contour map was developed using the pH measurements at the specific sampling locations thus, the contours and isolines were created using interpolation between data points. You should not necessarily use the map to document the pH at other particular map locations, but rather, use the map as a general indicator of pH throughout the country.
Note: This map shows one point in time, and since 2002 there has been a general reduction in things that cause acid rain. A newer map might look very different than this one. Still, lower precipitation pH values still will occur in the northeastern U.S.
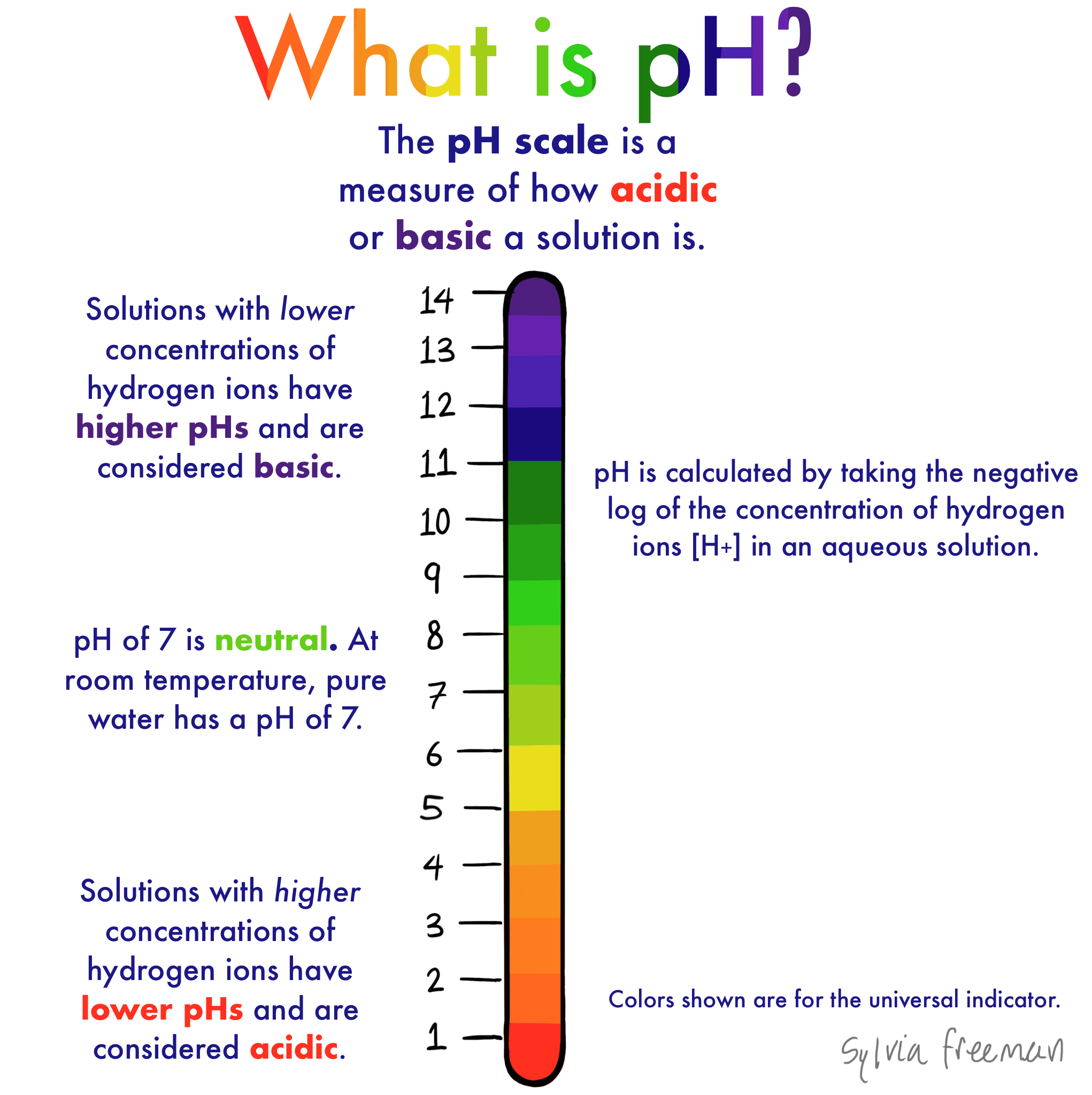
Ph Levels Below 7 Are Acidic While Levels Above 7 Are Alkaline
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
pH is a measure of hydrogen ion concentration, a measure of the acidity or alkalinity of a solution. The pH scale usually ranges from 0 to 14. Aqueous solutions at 25°C with a pH less than 7 are acidic, while those with a pH greater than 7 are basic or alkaline. A pH level of 7.0 at 25°C is defined as “neutral” because the concentration of H3O+ equals the concentration of OH in pure water. Very strong acids might have a negative pH, while very strong bases might have a pH greater than 14.
Don’t Miss: Practice 2 4 Reasoning In Algebra Answer Key
What Youll Learn To Do: Demonstrate Familiarity With The Ph Scale
Most people are familiar with the words acid and acidicwhether its because of acid rain or acidic foods like lemon juice. However, fewer people are aware of acids opposite: base . Basic substances include things like baking soda, soap, and bleach. Distilled water is a neutral substance. The pH scale, which measures from 0 to 14, provides an indication of just how acidic or basic a substance is.
Most parts of our body measure around 7.2 and 7.6 on the pH scale . If foreign strong substances dramatically change this pH, our bodies can no longer function properly.
In this outcome, well learn about acids and bases, and what impact they can have on living systems.
Why Ph Measurements Are Important
Chemicals reactions in water are affected by the acidity or alkalinity of the solution. This is important not only in the chemistry lab, but in industry, cooking, and medicine. pH is carefully regulated in human cells and blood. The normal pH range for blood is between 7.35 and 7.45. Variation by even a tenth of a pH unit may be fatal. Soil pH is important for crop germination and growth. Acid rain caused by natural and man-made pollutants changes the acidity of soil and water, greatly affecting living organisms and other processes. In cooking, pH changes are used in baking and brewing. Since many reactions in everyday life are affected by pH, it’s useful to know how to calculate and measure it.
Also Check: Who Are Paris Jackson’s Biological Parents
How Ph Is Measured
There are multiple methods of measuring pH.
- The most common method is a pH meter, which involves a pH-sensitive electrode and a reference electrode.
- Acid-base indicators change color in response to different pH values. Litmus paper and pH paper are used for quick, relatively imprecise measurements. These are strips of paper that have been treated with an indicator.
- A colorimeter may be used to measure the pH of a sample. A vial is filled with a specimen and a reagent is added to produce a pH-dependent color change. The color is compared against a chart or standard to determine the pH value.
Iupac Definition Of Ph
The International Union of Pure and Applied Chemistry has a slightly different pH scale that is based on electrochemical measurements of a standard buffer solution. Essentially, the definition uses the equation:
pH = -log aH+
where aH+ stands for hydrogen activity, which is the effective concentration of hydrogen ions in a solution. This might be slightly different from the true concentration. The IUPAC pH scale also includes thermodynamic factors, which may influence pH.
For most situations, the standard pH definition is sufficient.
You May Like: Paris Jackson Biological Father
What Is The Ph Scale
The pH scale measures how acidic or basic a substance is. The pH scale ranges from 0 to 14. A pH of 7 is neutral. A pH less than 7 is acidic.
Why pH is important in biology?
pH is important because substances such as our stomach acids tend to be at a certain pH in order to work properly. pH is also important because it must be at certain levels in order for living organisms to survive.
How Does Ph Affect Enzymes
Each and every enzyme is characterized by an optimum pH. At this specific pH level, a particular enzyme catalyzes the reaction at the fastest rate than at any other pH level. For example, the enzyme pepsin is most active at an acidic pH, whereas the enzyme trypsin performs best at a slightly alkaline pH. Thus, the optimum pH of an enzyme is different from that of another enzyme.
When we study pH, it is clearly defined as the measurement for the acidic or alkaline nature of a solution. To be more precise, pH indicates the concentration of dissolved hydrogen ions in the particular solution. An increase or decrease in the pH changes the ion concentration in the solution.
These ions alter the structure of the enzymes and at times the substrate, either due to formation of additional bonds or breakage of already existing bonds. Ultimately, the chemical makeup of the enzyme and substrate are changed. Also, the active site of the enzyme is changed, after which the substrate can no longer identify the enzyme. For more information on enzymes, you can refer to enzyme substrate complex.
Also Check: Why Are There Different Branches Of Chemistry
Strong Acids And Bases
Strong acids and bases are compounds that, for practical purposes, are completely dissociated in water. Under normal circumstances this means that the concentration of hydrogen ions in acidic solution can be taken to be equal to the concentration of the acid. The pH is then equal to minus the logarithm of the concentration value. Hydrochloric acid is an example of a strong acid. The pH of a 0.01M solution of HCl is equal to log10, that is, pH = 2. Sodium hydroxide, NaOH, is an example of a strong base. The p value of a 0.01M solution of NaOH is equal to log10, that is, p = 2. From the definition of p above, this means that the pH is equal to about 12. For solutions of sodium hydroxide at higher concentrations the self-ionization equilibrium must be taken into account.
Self-ionization must also be considered when concentrations are extremely low. Consider, for example, a solution of hydrochloric acid at a concentration of 5×108M. The simple procedure given above would suggest that it has a pH of 7.3. This is clearly wrong as an acid solution should have a pH of less than 7. Treating the system as a mixture of hydrochloric acid and the amphoteric substance water, a pH of 6.89 results.
Who Discovered The Ph
Exactly 100 years ago, Carlsberg s director of chemistry, Søren Sørensen, developed a vital diagnostic tool for measuring acidity, thus helping to detect digestive , respiratory and metabolic disorders. The invention of Sørensen was the pH scale.
For detailed discussions on pH of acids and bases and its importance, Download BYJUS The Learning App.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
Don’t Miss: What Does P Stand For In Math
What Causes High Ph In Water
The cause of the unbalanced pH is the soil, bedrock, or other underlying composition from which the water source comes. High alkaline water is a consequence of rocky areas with a lot of calcareous. It contains compounds of carbonate, bicarbonate, and hydroxide that dissolve and migrate with the water, increasing its pH.
Unified Absolute Ph Scale
In 2010, a new “unified absolute pH scale” has been proposed that would allow various pH ranges across different solutions to use a common proton reference standard. It has been developed on the basis of the absolute chemical potential of the proton. This model uses the Lewis acidbase definition. This scale applies to liquids, gases and even solids.
Don’t Miss: Which Founding Contributors To Psychology Helped Develop Behaviorism
Buffers Ph Acids And Bases
pHacidityalkalinity.
The pH scale ranges from 0 to 14. A change of one unit on the pH scale represents a change in the concentration of hydrogen ions by a factor of 10, a change in two units represents a change in the concentration of hydrogen ions by a factor of 100. Thus, small changes in pH represent large changes in the concentrations of hydrogen ions. Pure water is neutral. It is neither acidic nor basic, and has a pH of 7.0. Anything below 7.0 is acidic, and anything above 7.0 is alkaline . The blood in your veins is slightly alkaline . The environment in your stomach is highly acidic . Orange juice is mildly acidic , whereas baking soda is basic .
Figure 1
Acids are substances that provide hydrogen ions and lower pH, whereas bases provide hydroxide ions and raise pH. The stronger the acid, the more readily it donates H+. For example, hydrochloric acid and lemon juice are very acidic and readily give up H+ when added to water. Conversely, bases are those substances that readily donate OH. The OH ions combine with H+ to produce water, which raises a substances pH. Sodium hydroxide and many household cleaners are very alkaline and give up OH rapidly when placed in water, thereby raising the pH.
What Is The Ph Scale In Biology
pHpHpHpH scalepH
. In respect to this, what is pH biology?
PH, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. The term, widely used in chemistry, biology, and agronomy, translates the values of the concentration of the hydrogen ionwhich ordinarily ranges between about 1 and 1014 gram-equivalents per litreinto numbers between 0 and 14.
Furthermore, what is the use of pH scale? t?/) is a scale used to specify how acidic or basic a water-based solution is. Acidic solutions have a lower pH, while basic solutions have a higher pH. At room temperature , pure water is neither acidic nor basic and has a pH of 7.
Likewise, people ask, what is the pH scale?
The pH scale measures how acidic or basic a substance is. The pH scale ranges from 0 to 14. A pH of 7 is neutral. A pH less than 7 is acidic.
Why pH is important in biology?
pH is important because substances such as our stomach acids tend to be at a certain pH in order to work properly. pH is also important because it must be at certain levels in order for living organisms to survive.
Recommended Reading: Which Founding Contributors To Psychology Helped Develop Behaviorism
Monitoring Culture Medium Ph Under Incubation
Fig. 1
Measuring and setting medium pH under incubation. a Absorbance spectrum of Phenol Red in Dulbeccos modified Eagles medium with 10% foetal bovine serum , 1% penicillinstreptomycin , 10mM HEPES -1-piperazineethanesulfonic acid) plus 10mM 2–ethanesulfonic acid , and titrated to the indicated pH. Arrows indicate wavelengths for optimal ratiometric analysis. b pH dependence of 560/430nm ratio, fitted to curve: pH=8.35+log)/). c Controlling equilibrium pH by varying pCO2 and in DMEM supplemented with 10% FBS and 1% PS. Dashed line plots Eq. . Continuous line is best fit to Eq. , which accounts for buffering by serum . Inset replots the data at low . d Empirical determination of intrinsic buffering capacity of DMEM nominally lacking buffers titration with either HCl or NaOH. Inverse of slope provides an estimate of buffering due to serum proteins and media salts. All measurements were repeated three times . Data are shown as mean±SEM
Effect Of Ph On Enzymes
For every enzyme, there is an optimum pH value, at which the specific enzyme functions most actively. Any change in this pH significantly affects the enzyme activity and/or the rate of reaction. To know more about the relation between pH and enzymes, and/or the effect of pH on enzymes, go through this write-up.
Like it? Share it!
For every enzyme, there is an optimum pH value, at which the specific enzyme functions most actively. Any change in this pH significantly affects the enzyme activity and/or the rate of reaction. To know more about the relation between pH and enzymes, and/or the effect of pH on enzymes, go through this write-up.
Enzymes are proteinaceous catalysts, which speed up the rate of a biochemical reaction. They reduce the activation energy that is essential for starting any type of chemical reaction. With a low energy requirement for activation, the reaction takes place faster. The overall performance of an enzyme depends on various factors, such as temperature, pH, cofactors, activators, and inhibitors. You might have a fair idea regarding the effect of pH on enzymes. But why and how does pH and temperature affect enzymes?
You May Like: Holt Geometry Lesson 4.5 Practice B Answers
Stability Of Co2/hco3 Buffering
In most instances, media are prepared to a neutral or alkaline pH, and over this pH range, the HendersonHasselbalch equation is adequate for predicting equilibrium pH. However, the robustness of Eq. depends on the accuracy of pCO2 and measurements. In many instances, it may be appropriate to assume that the amount of HCO3 salt added to a medium accurately predicts the final concentration of base however, some formulations contain weak acids that react with HCO3 salts. Under these circumstances, Eq. will underestimate pH, and therefore direct pH measurements are advocated. For example, the addition of 22mM of NaHCO3 to media supplemented with lactic acid will not produce the expected pH of 7.4 due to the titration reaction . If, instead, media contained a salt of lactic acid , then the acid-titration reaction with HCO3 will not take place, and Eq. adequately approximates pH . The difference in behaviour between lactic acid and its conjugate base can be explained in terms of equilibria:
Around neutral pH, this equilibrium is shifted far to the right. After dissolving a lactate salt, only a tiny fraction of lactate will protonate, thus the change in pH is negligible. In contrast, lactic acid added to a medium undergoes near-complete deprotonation, which reduces pH.
Fig. 2