How To Calculate W/v
When making a solution in a lab, there are different ways to express the concentration. Some of the most common ways are listed below:
Here, the focus will be specifically on how to make solutions with their concentrations expressed as weight by volume or w/v.
Kw Increases With Increase Of Temperature
Autoionisation of water is an endothermic process. According to Le chateliers principle, if conditions are changed in a equilibrium process, the equilibrium will shift to such a direction where it can minimize the effect of the change of the condition. Thus if water is heated the equilibrium will shift to right to form more ions by absorbing extra heat as this is an endothermic process. According to the equation of Kw, if the concentration of ions increases the Kw increases. So we can say that Kw increases with the increase of temperature.
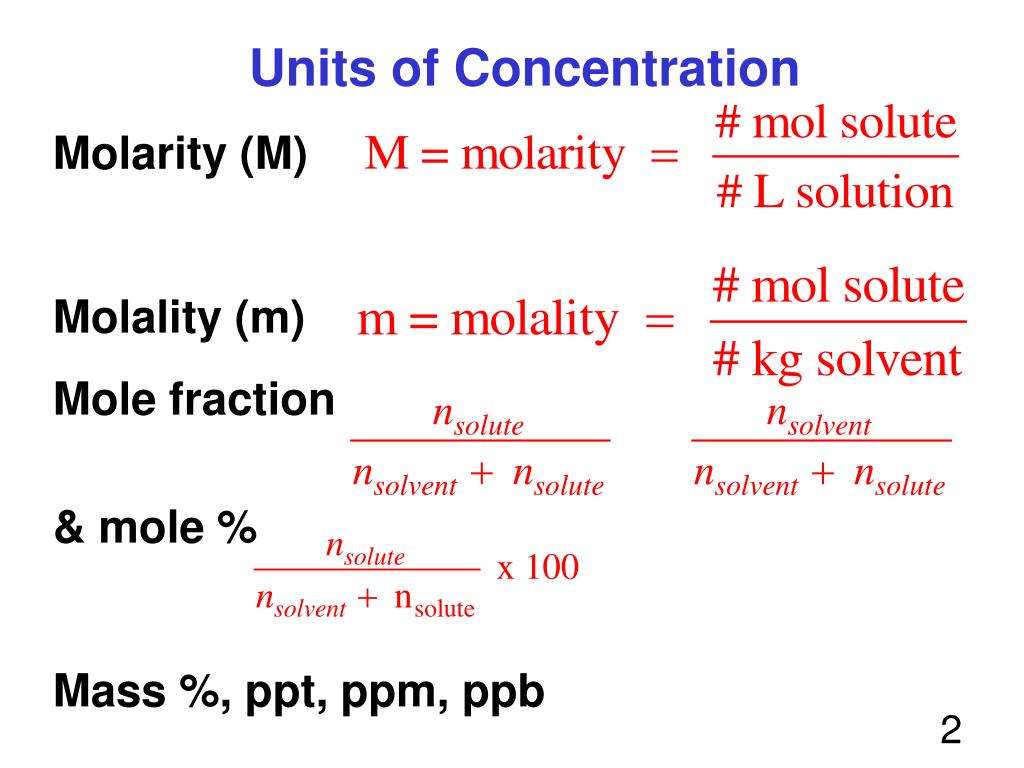
Recall that, in general, concentration tells you how much solute is present in a solution.
concentration = amount of solute ÷amount of solution
A concentration in parts per million may refer to the mass of solute present in the volume of solution or it may refer to the mass of solute present in a mass of solution .
In SI units, w/w concentration would be given in kilograms of solute per kilograms of solution. So, a 1 part per million solution would be 1 kg of solute per 1 million kilograms of solution. And these masses are just too large to be useful in Chemistry laboratory. But we can divide the masses of solute and solution by 1 million to arrive at more useful units:
| 1 ppm |
ppm = mass of solute ÷ mass of solution
ppm = mass of solute ÷ mass of solution
You should practice rearranging the equations above in order to find mass of solute, volume of solution or mass of solution:
What Do S M W M
I am referring to Donald Pavia’s Introduction to Spectroscopy, and I found this table that tells about the intensities of the peaks of vibrations of certain bonds.
I can’t understand what s, m, w, w-s, etc. are supposed to mean. My guess is that s could mean small, m could mean medium and w could mean weak. But that’s crazy, since small and weak mean the same thing? So what do they mean actually?
- 3Jun 27, 2017 at 15:44
- 1$\begingroup$@hBy2Py is correct. I have found a similar table on another page which is a bit more explicit in what its abbreviations mean and every thing lines up with the the notation of strong/ medium/ weak. www2.chemistry.msu.edu/faculty/reusch/virttxtjml/Spectrpy/$\endgroup$Jun 27, 2017 at 16:54
- 2$\begingroup$It is described in the book, p 28 “Often, when reading the literature of organic chemistry, you will find absorptions referred to as strong , medium , weak , broad, or sharp.”$\endgroup$
You May Like: How Much Physics Is On The New Mcat
Molecular Weight And Isotopes
Note, if you are working with specific isotopes of an atom, you should use the atomic weight of that isotope rather than the weighted average provided from the periodic table. For example, if instead of hydrogen, you are dealing only with the isotope deuterium, you use 2.00 rather than 1.01 for the atomic mass of the element. Ordinarily, the difference between the atomic weight of an element and the atomic weight of one specific isotope is relatively minor, but it can be important in certain calculations!
What Does The Ww Mean
In an effort to shed the Wound tag, Weight Watchers International will change its name to a 2-letter acronym. CEO Mindy Grossman said there is no trademark on the letters because the company does not refer to its Weight Watchers product, nor is the company trademarked to speak of Wellness that Works.
Also Check: Test Form 2b Answers
How To Calculate Volume Percent Concentration
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
Volume percent or volume/volume percent is used when preparing solutions of liquids. It is very easy to prepare a chemical solution using volume percent, but if you misunderstand the definition of this unit of concentration, you’ll experience problems.
How Molecular Weight Is Determined
Empirical data on the molecular weight of a compound depends on the size of the molecule in question. Mass spectrometry is commonly used to find the molecular mass of small to medium-sized molecules. The weight of larger molecules and macromolecules is found using light scattering and viscosity. Specifically, the Zimm method of light scattering and the hydrodynamic methods dynamic light scattering , size-exclusion chromatography , diffusion-ordered nuclear magnetic resonance spectroscopy , and viscometry may be used.
Recommended Reading: What Type Of Math Do Architects Use
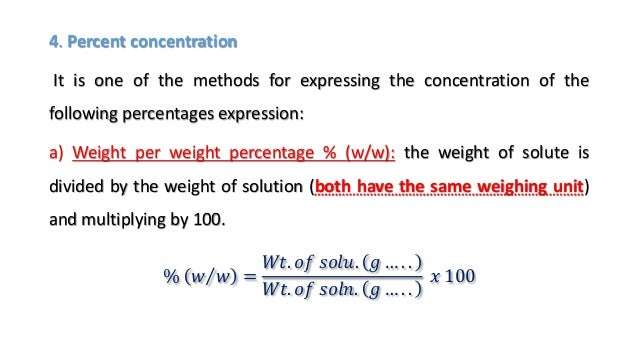
Percent By Weight Formula
Solutions can be described in other concentration besides molarity, normality or molality. Solutions are sometimes represented in terms of relative per cent concentration of solute in a solution. To determine the weight per cent of a solution, divide the mass of solute by mass of the solution and multiply by 100 to obtain per cent.
The per cent by weight formula can be expressed as
Example 1
Calculate how many grams of NaOH are required to make a 30.0% solution by using De-ionized water as the solvent.
Solution
Substitute the numbers that are known in the basic formula for per cent weight
X g of solute in 100g of solution = 30.0%
30.0 g of NaOH = X
Thus, 30 grams of NaOH must be dissolved in 70 grams of de-ionized water to make a 30% solution of NaOH in water. Furthermore, any amount of NaOH can be dissolved in de-ionized water in a 3:7 ratio to form a 30% solution of NaOH.
Example 2
Determine the % of a solution which has 25.0g of NaCl dissolved in100mL of De-ionized water.
Solution
Therefore, mass of solution = mass of solute + mass of solvent = 100g + 25g = 125g
Mass % of NaCl in this solution = *100% = 20%
Therefore, mass % of NaCl in the solution is 20%
Why Serial Dilution Method Is Important
Serial dilutions are used to accurately create extremely diluted solutions, as well as solutions for experiments that require a concentration curve with an exponential or logarithmic scale. Serial dilutions are widely used in experimental sciences, including biochemistry, pharmacology, microbiology, and physics.
Read Also: Theory Of Everything 2 All Coins
The Militarization Of Academic And Industrial Chemistry
World War I had numerous causes, including colonial competition, economic rivalry, and various ideological and cultural clashes among the rising nation states of Europe. A complex and binding system of alliances among the Central Powers and the Allied Powers placed peace in a delicate balance. The tipping point came on June 28, 1914, with the assassination of Archduke Franz Ferdinand of Austria-Hungary by a Serbian national. This single act set off a chain of events that quickly plunged the world into a global war that eventually claimed between 9 million and 10 million lives and lasted 4 years.6
The economic and industrial forces that altered the face of Europe during the first decade of the 20th century were also instrumental in creating many of the technological innovations driving the war. In time, tanks, submarines, and aircraft revolutionized how World War I was waged on land, sea, and in the air. Chemical weapons were another new marvel of the war, and their successful research, development, and deployment reflected the increasing sophistication of scientific and engineering practice. At the same time, physicians and medical researchers struggled to create adequate defensive systems and medical procedures to limit casualties. By the time of the armistice on November 11, 1918, the use of chemical weapons such as chlorine, phosgene, and mustard gas had resulted in more than 1.3 million casualties and approximately 90 000 deaths .
Introduction To The Water Ionization Constant Kw
Pure water undergoes auto-ionization or self-ionization by donating or accepting a proton between two molecules of water to form H3O+ and OH ions. This is also known as autoprotolysis or amphoteric nature of water.
The hydronium ion is a very strong acid and hydroxide ion is a very strong base. Thus they can associate again to form water molecule. So water molecules and the ions always stay in equilibrium. And the equilibrium lies to the left. Thus a very small amount of hydronium ions and hydroxide ions are found in water.
The equilibrium constant for this autoionisation of water is known as Kw. Thus
Kw =
Or simply Kw = .
Here we omit the concentration of water molecule which should stay as a denominator. The reason is, not much change in concentration is observed during this process.
You May Like: How To Find The Half Life
What Do Chemists Do
Chemists work in a variety of fields, including research and development, quality control, manufacturing, environmental protection, consulting and law. They can work at universities, for the government or in private industry, according to the ACS.
Here are some examples of what chemists do:
Research and development
In academia, chemists performing research aim to further knowledge about a particular topic, and may not necessarily have a specific application in mind. Their results, however, can still be applied to relevant products and applications.
In industry, chemists in research and development use scientific knowledge to develop or improve a specific product or process. For example, food chemists improve the quality, safety, storage and taste of food pharmaceutical chemists develop and analyze the quality of drugs and other medical formulations and agricultural chemists develop fertilizers, insecticides and herbicides necessary for large-scale crop production.
Sometimes, research and development may not involve bettering the product itself, but rather the manufacturing process involved in making that product. Chemical engineers and process engineers devise new ways to make the manufacturing of their products easier and more cost effective, such as increasing the speed and/or yield of a product for a given budget.
Environmental protection
Additional resources:
Working With Formula Weights

For example, the molecular weight of calcium chloride is 111.0 grams per mole , which is the same as the formula weight if the material is anhydrous. Calcium chloride dihydrate is 147.0 g/mol. For CaCl26H2O the formula weight is 219.1 g/mol.
A hydrated compound is a compound that is surrounded by water molecules that are held in place by hydrogen bonds. The water molecules in a hydrated compound become part of the solution when the material is dissolved. Thus, 111.0 grams of anhydrous CaCl2, 147.0 grams of dihydrated CaCl2, or 219.1 grams of CaCl2 hexahydrate in one liter final volume all give a 1 mole per liter solution, abbreviated 1M.
Suppose that you need one liter of a solution of 10 mM calcium chloride , and suppose that you have only CaCl2 dihydrate. To make your 10 mM solution you weigh out 1/100 of the formula weight for dihydrated CaCl2, which is 0.01 x 147.0 = 1.47 grams and bring it to one liter.
Recommended Reading: Paris Jackson’s Father
Industrial Workers Cope With Chemical Agents
The scale of industrial chemical production during the course of the war was enormous and without precedent. Chemical companies, universities, and government laboratories from all the warring nations labored at great cost to produce chemicals not only for traditional war materiel such as munitions and fuel but also for a new generation of weapons. The production of certain chemical weapons was complex and often involved the synthesis of various chemical precursors needed for the completion of a specific chemical agent. Estimates by historians and military officials place production in excess of 124200 tons of gas, indicating a substantial investment on the part of various governments and chemical manufacturers to meet expansive wartime production schedules.53 While thoughts of gas warfare usually bring to mind images of the battlefield, the risks and sacrifices workers made in their home countries to manufacture war gases was no less valuable and in many ways presented just as many, if not more, health risks.
Molecular Weight Versus Molecular Mass
Molecular weight is often used interchangeably with molecular mass in chemistry, although technically there is a difference between the two. Molecular mass is a measure of mass and molecular weight is a measure of force acting on the molecular mass. A more correct term for both molecular weight and molecular mass, as they are used in chemistry, would be relative molecular mass.
You May Like: Who Are Paris Jacksons Biological Parents
Read Also: Mood Congruent Memory Definition Psychology
When Is Volume Percent Used
Volume percent should be used whenever a solution is prepared by mixing pure liquid solutions. In particular, it’s useful where miscibility comes into play, as with volume and alcohol.
Acid and base aqueous reagents are usually described using weight percent . An example is concentrated hydrochloric acid, which is 37% HCl w/w. Dilute solutions are often described using weight/volume % . An example is 1% sodium dodecyl sulfate. Although it’s a good idea to always cite the units used in percentages, it seems common for people to omit them for w/v%. Also, note “weight” is really mass.
What Is A 1 To 20 Dilution
These two components proportionally combine to create a dilution. For example, a 1:20 dilution converts to a 1/20 dilution factor. Multiply the final desired volume by the dilution factor to determine the needed volume of the stock solution. In our example, 30 mL x 1 ÷ 20 = 1.5 mL of stock solution.
Don’t Miss: Holt Mcdougal Geometry: Practice And Problem Solving Workbook Answer Key
S Per Million And Parts Per Billion
Very low solute concentrations are often expressed using appropriately small units such as parts per million or parts per billion . Like percentage units, ppm and ppb may be defined in terms of masses, volumes, or mixed mass-volume units. There are also ppm and ppb units defined with respect to numbers of atoms and molecules.
The mass-based definitions of ppm and ppb are given here:
Both ppm and ppb are convenient units for reporting the concentrations of pollutants and other trace contaminants in water. Concentrations of these contaminants are typically very low in treated and natural waters, and their levels cannot exceed relatively low concentration thresholds without causing adverse effects on health and wildlife. For example, the EPA has identified the maximum safe level of fluoride ion in tap water to be 4 ppm. Inline water filters are designed to reduce the concentration of fluoride and several other trace-level contaminants in tap water .
Figure 3.
Using %w/w Percentage Weight Concentrations In Cosmetic Formulas
To use Product Manager it is important that you understand the principle of entering %w/w amounts in your formulas composition. This is the most universally accepted method for formulation of cosmetic products and the method that is used in Product Manager.
In percent solutions, the weight of a solute is expressed as a percentage of the total solution in weight. By solute we mean, a raw material that is used in your formula, whereas solution refers to the total resultant mixture of your product.
Read Also: Beth The Psychopathic Child Now
Worked Examples: W/v% Calculations Requiring Unit Conversions
Question 1.
What is the weight/volume percentage concentration of this solution in g/100 mL?
Solution:
Step 1: Write the equation: either w/v% = w/v × 100 or m/v% = m/v × 100
Step 2: Identify the solute and solvent
solvent is water, H2O, because this is an aqueous solution.
Step 3: Extract the data from the question
mass KCl = 45.0 g
volume of solution = 2.00 L
Step 4: Check the units for consistency and convert if necessary
mass KCl = 45.0 g
volume of solution = 2.00 L V = 2.00
Step 5: Substitute these values into the equation and solve.
w/v = × 100
w/v = × 100 = 2.25 g/100 mL
Step 6: Write the answer
w/v% = 2.25 g/100 mL = 2.25 % = 2.25 %
Question 2.
What is the mass/volume percentage concentration of this solution in g/100 mL?
Solution:
Step 1: Write the equation: either m/v% = m/v × 100 or w/v% = w/v × 100
Step 2: Identify the solute and solvent
solvent is water, H2O, because this is an aqueous solution.
Step 3: Extract the data from the question
mass solute = 750 mg
volume solution = 15 mL
Step 4: Check the units for consistency and convert if necessary
mass solute = 750 mg mass solute = 750
volume solution = 15 mL
Step 5: Substitute these values into the equation and solve.
w/v = × 100
w/v = × 100 = 5.0 g/100 mL
Step 6: Write the answer
m/v% = 5.0 g/100 mL = 5.0 % = 5.0 %
Question 3.
What is the weight/volume percentage concentration of this solution in g/100 mL?
Solution:
Step 1: Write the equation: either w/v% = w/v × 100 or m/v% = m/v × 100
mass solute = 1.15 kg
Work Definition In Chemistry
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
The word work means different things in different contexts. In science, it is a thermodynamic concept. The SI unit for work is the joule. Physicists and chemists, in particular, view work in relation to energy:
Also Check: Geometry Segment Addition Postulate Worksheet
Recommended Reading: Does Elton John Have Biological Children
Reagent Volume And Mass Calculations
In the sections above we calculated the w/v% concentration of solutions using the known mass of solute and volume of solution.
When we come to use this solution in the lab, we are most likely to use a pipette or burette to deliver a volume of solution.
If we know the volume of solution used, we can calculate the mass of solute present.
| mass of solute = | volume of solution × w/v 100 |
If we know the mass of solute we want to use, we can calculate the volume of solution we will need to use.
| volume of solution = |
solvent is water, H2O, because this is an aqueous solution.
Step 3: Extract the data from the question
mass of solute required = mass = 1.22 g
concentration of NaCl provided = w/v = 11.78 g/100 mL
volume of solution needed = ? mL
Step 4: Check the units for consistency and convert if necessary
mass of solute required = mass = 1.22 g
concentration of NaCl provided = w/v = 11.78 g/100 mL
volume of solution needed = ? mL
Step 5: Substitute these values into the equation and solve.
| volume of solution |
Step 6: Write the answer
volume of solution = 10.4 mL
Question 2.
Solution:
Step 1: Write the equation: either w/v% = w/v × 100 or m/v% = m/v × 100
Rearrange the equation to find mass of solute:
w/v = mass solute ÷ volume solution × 100
Divide both sides of the equation by 100
w/v ÷ 100 = mass solute ÷ volume solution
Multiple both sides of the equation by volume solution
mass solute = ÷ 100) × volume solution
Step 2: Identify the solute and solvent
mass solute = mass = ? g