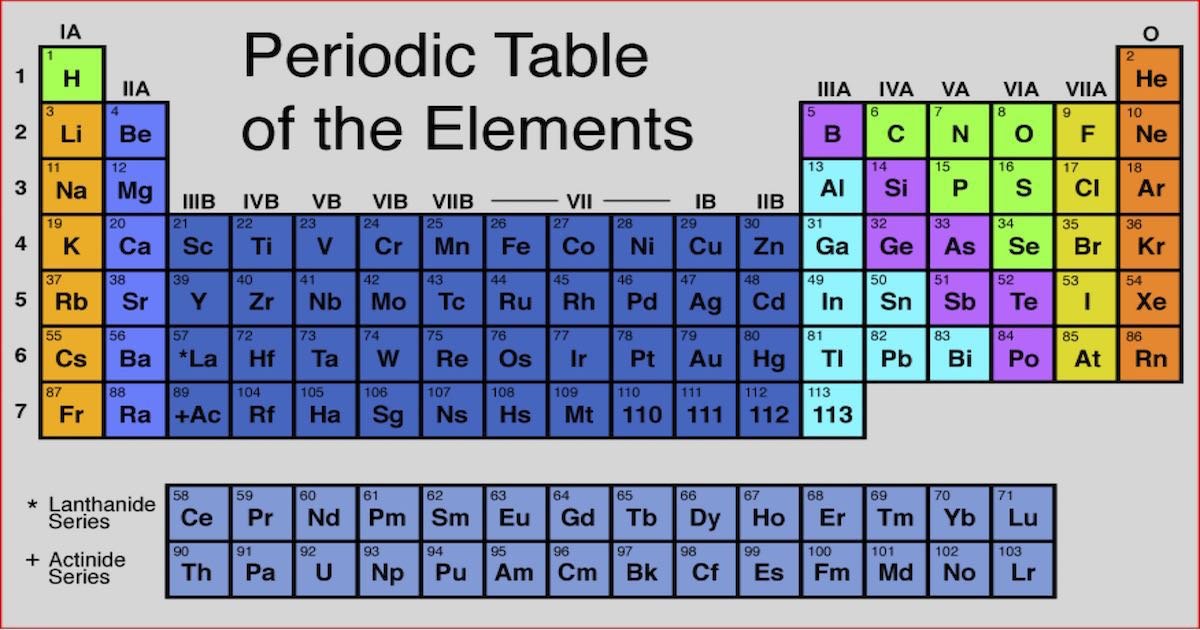
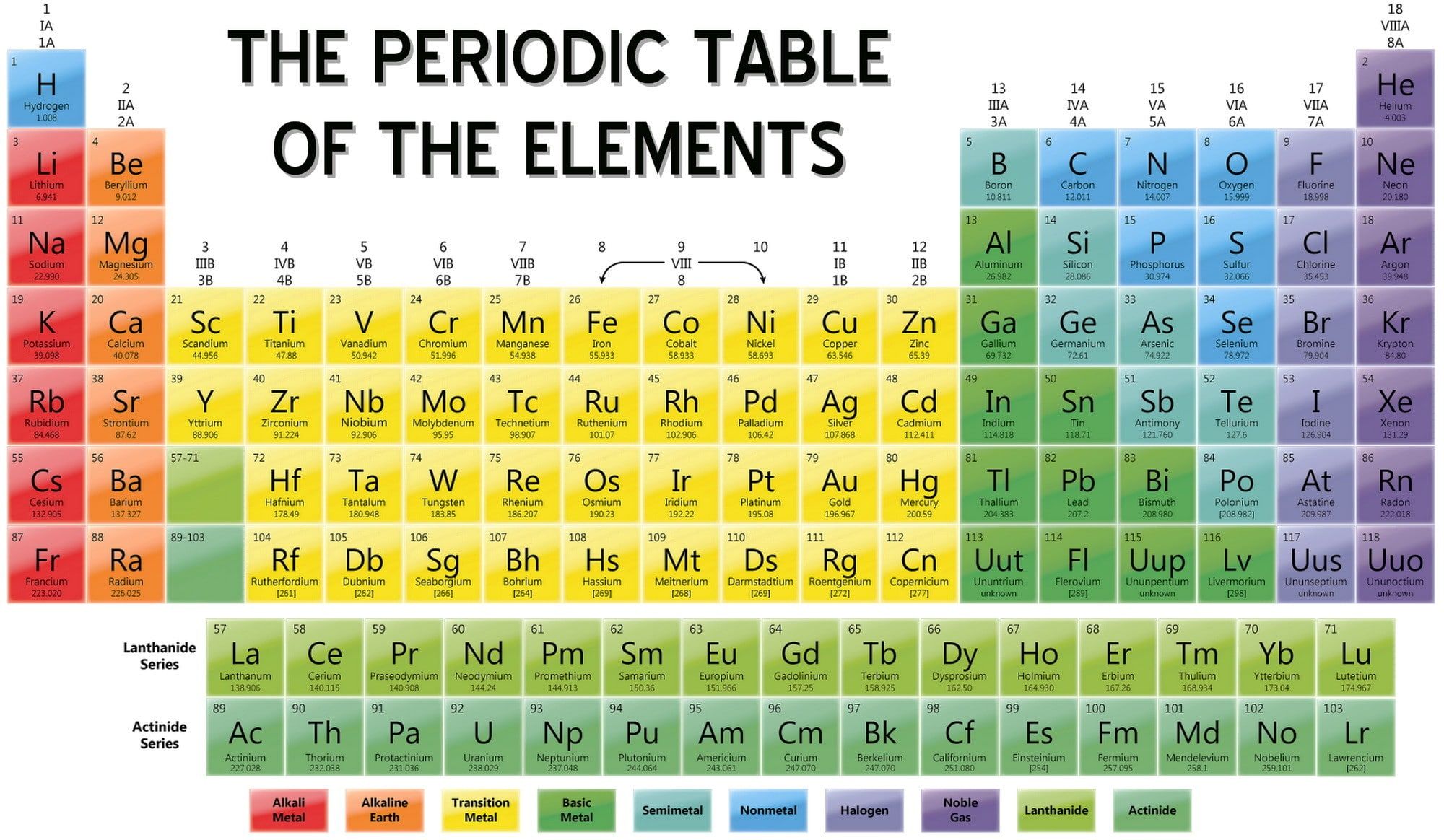
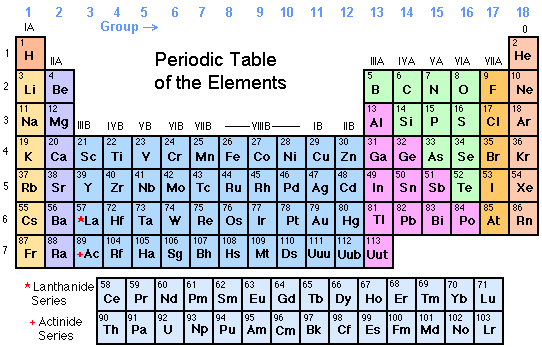
Worked Example: Use Equilibrium Constant Values At Different Temperatures To Predict Relative Value Of H
Question: Consider the synthesis of I3- from the reactants I2 and I- as shown by the following chemical equation:
I2 + I- I3-
The reaction was carried out at a number of different temperatures and the equilibrium constant, Kc, was calculated for each temperature as shown in the table below:
| Temperature |
|---|
Endothermic reaction: Kc increases as temperature increases
Exothermic reaction: Kc decreases as temperature increases
Determine whether the reaction is exothermic or endothermic
From the data in the table, as temperature increases from 0°C to 100°C, the value of Kc decreases from 1360 to 190.
As temperature increases, Kc decreases.
This reaction is exothermic.
Is your answer plausible?
Assume the reaction is exothermic and predict the effect of increasing temperature on the value of Kc
We can see that K’ will be a smaller number than K because:
- the value of the numerator has decreased from to – x
- and the value of the denominator has increased from to
- and a smaller number divided by a larger number results in a smaller number
For an exothermic reaction the value of the equilibrium constant decreases when temperature increases, which is what we saw in the data in the question, so we are confident are answer is correct.
State your solution to the problem.
I2 + I- I3- is an exothermic reaction.
Can you apply this?
Chambers 20th Century Dictionaryrate This Definition:
Chemistry
kemis-tri, formerly Chymistry, n. the science which treats of the properties of substances both elementary and compound, and of the laws of their combination and action one upon another.adjs.Chemic, -al , Chemiatric .adv.Chemically.n.pl.Chemicals, substances which form the subject of chemical effects.ns.Chemism, chemical action Chemist, one skilled in chemistry, specially a druggist or apothecary.Chemical affinity, the name given to the tendency to combine with one another which is exhibited by many substances, or to the force by which the substances constituting a compound are held together Chemical notation, a method of expressing the composition of chemical substances and representing chemical changes, by certain known symbols and formulæ Chemical works, manufactories where chemical processes are carried on for trade, as alkali works, & c.
Cosmic Formation And Distribution
Potassium is formed in supernovae by nucleosynthesis from lighter atoms. Potassium is principally created in Type II supernovae via an explosive oxygen-burning process.40K is also formed in s-process nucleosynthesis and the neon burning process.
Potassium is the 20th most abundant element in the solar system and the 17th most abundant element by weight in the Earth. It makes up about 2.6% of the weight of the earth’s crust and is the seventh most abundant element in the crust. The potassium concentration in seawater is 0.39 g/L , about one twenty-seventh the concentration of sodium.
You May Like: What Math Class Do 9th Graders Take
Potassium Element In Human Body
Potassium is a critical element for the human body. Specifically, potassium ions are present in conjunction with a wide variety of proteins and enzymes. It helps to maintain acidity levels and blood pressure. It is the predominant positive ion inside human cells, sodium being the major positive ions outside human cells. The difference between the concentrations of these two ions inside and outside can make a difference in electric potential, which is essential for basic body functions such as neurotransmission, heart function, and muscle contraction.
Health Effects Of Potassium
Potassium can be found in vegetables, fruit, potatoes, meat, bread, milk and nuts. It plays an important role in the physical fluid system of humans and it assists nerve functions. Potassium, as the ion K+, concnetrate inside cells, and 95% of the body’s potassium is so located. When our kidneys are somehow malfunctioning an accumulation of potassium will consist. This can lead to disturbing heartbeats.
Potassium can effect you when breathed in. Inhalation of dust or mists can irritate the eyes, nose, throat, lungs with sneezing, coughing and sore throat. Higher exposures may cause a build up of fluid in the lungs, this can cause death. Skin and eye contact can cause severe burns leading to permanent damage.
Recommended Reading: What Does Consistent Mean In Algebra
Princeton’s Wordnetrate This Definition:
chemistry, chemical sciencenoun
the science of matter the branch of the natural sciences dealing with the composition of substances and their properties and reactions
chemistrynoun
the chemical composition and properties of a substance or object
“the chemistry of soil”
chemistry, interpersonal chemistry, alchemynoun
the way two individuals relate to each other
“their chemistry was wrong from the beginning — they hated each other” “a mysterious alchemy brought them together”
Chemistry Definitions Starting With The Letter K
This chemistry dictionary offers the chemistry definitions starting with the letter K. These glossary terms are commonly used in chemistry and chemical engineering. Click the letter below to find the terms and definitions beginning with that letter.
K-electron K-electron refers to the electrons of an atom with energy quantum number n = 1.Also known as: K electron
K-shell K-shell refers to the electrons of an atom with energy quantum number n = 1.Also known as: K shell
kalium Kalium is the German name for the element potassium. Kalium is the source of the symbol K for potassium on the periodic table.
Kelvin effect The Kelvin effect is the generation or absorption of heat when an electrical current is passed through a material.
Kelvin temperature scale The Kelvin temperature scale is an absolute temperature scale based on the definition that the volume of a gas at constant pressure is directly proportional to temperature and that 100 degrees separate the freezing and boiling points of water. Usage: Kelvin temperatures are written with a capital letter K and without the degree symbol, such as 1 K, 1120 K. Note that 0 K is absolute zero and there are no negative Kelvin temperatures.
ketal A ketal is a type of acetal compound with general structure R2C2 where neither R group is a hydrogen atom. Ketals are a subclass of acetal compounds derived from ketones.
ketoheptose A ketoheptose is a heptose carbohydrate that contains a ketone functional group.
You May Like: 6 Major Branches Of Chemistry
Balanced Or Complete Fertilizers
Complete fertilizers or so-called balanced fertilizers have equal amounts of NPK. A fertilizer listed as “10-10-10” would be considered a balanced fertilizer because of the equal proportions, while one listed as “10-0-10” would not be considered complete, but referred to as an “incomplete fertilizer.”
An incomplete fertilizer is not necessarily inferior to a complete fertilizer. Identifying the right fertilizer for your needs depends on a variety of circumstances. If your soil already has an excess of one of the three nutrients in NPK, you could actually be harming some of your plants by adding more of it to the soilin this case, an incomplete fertilizer may actually be the right choice for you. It’s yet another reason why it’s so important to have your soil tested. Otherwise, whenever you add anything to your soil, the effect is left to chance.
What Is Atm Implied Volatility
A tool that measures the calculated or implied mid-rate volatility for an ATM option for a specific expiration date. Using the Black-Scholes model, the ATM volatility can be defined as the volatility value that makes the implied price of an ATM vanilla option equal to the market price of that option.
Read Also: What Does The Denominator Tell You
What Does K Stand For In Chemistry Equilibrium
4.2/5isKisreactionequilibriumK isreactionequilibrium is
Also asked, what is K at equilibrium?
If K is larger than 1, the mixture contains mostly products. K< 1. If K is less than 1, the mixture contains mostly reactants. K = 1. If K is about equal to 1, the reaction will reach equilibrium as an intermediate mixture, meaning the amounts of products and reactants will be about the same.
Also, what is equilibrium constant in chemistry? Definition of equilibrium constant. : a number that expresses the relationship between the amounts of products and reactants present at equilibrium in a reversible chemical reaction at a given temperature.
Considering this, what does K stand for in chemistry?
Potassium. The name is derived from the english word potash. The chemical symbol K comes from kalium, the Mediaeval Latin for potash, which may have derived from the arabic word qali, meaning alkali.
Which K values would indicate?
Explanation: If the value of K is less than one then there is more reactant than product at equilibrium and if the value of K is more than one than there is more product than reactant at equilibrium.
The Importance Of Npk
Not all types of plants have the same nutrient requirements, and you can sometimes do more harm than good when applying supplements haphazardly. Applying a fertilizer high in nitrogen will cause certain plants to put all their energy into producing foliage at the expense of flowers.
If you have not had your soil tested and do not have a good grasp of how well your soil is meeting the nutritional needs of a plant but still feel the need to feed it at a particular time, you should:
- Use compost instead of chemical fertilizer.
- Apply a slow-release fertilizer, which is less likely to harm plants to any great degree.
You May Like: Who Is Khloe Kardashian’s Real Dad
Worked Example: Predict The Value For An Equilibrium Constant K At A Different Temperature
Question: The decomposition of N2O4 to produce NO2 is an endothermic chemical reaction which can be represented by the following chemical equation:
N2O4 2NO2
At 25°C the value of the equilibrium constant, Kc is 4.7 × 10-3.
Predict whether the equilibrium constant for this reaction at 100°C will be greater than, less than, or equal to 4.7 × 10-3.
Solution:
| STOP |
K’ will be larger than K because:
- the value of the numerator has increased from 2 to 2
- and the value of the denominator has decreased from to
- and a larger number divided by a smaller number will give a larger number
K’ will be greater than 4.7 × 10-3 so our answer is plausible.
State your solution to the problem.
The value of the equilibrium constant will be greater than 4.7 × 10-3 at 100°C.
Can you apply this?
Take the exam now!
The Use Of Dots In Phase Diagrams
Let’s take the example of the ternary phase diagram of $\ce$,$\ce$,$\ce$. The formulas ofthe various resulting complex sodium aluminosilicatesare all expressed in the form $\ce$in which it is implicitly understood that these initial starting components do not exist as such within the resulting compounds. They represent the combining ratios of the binary/ternary starting materials required for the synthesis of the compound.
Don’t Miss: What Are Two Types Of Elastic Forces
Webster Dictionaryrate This Definition:
Chemistrynoun
that branch of science which treats of the composition of substances, and of the changes which they undergo in consequence of alterations in the constitution of the molecules, which depend upon variations of the number, kind, or mode of arrangement, of the constituent atoms. These atoms are not assumed to be indivisible, but merely the finest grade of subdivision hitherto attained. Chemistry deals with the changes in the composition and constitution of molecules. See Atom, Molecule
Etymology:
Chemistrynoun
an application of chemical theory and method to the consideration of some particular subject as, the chemistry of iron the chemistry of indigo
Etymology:
Chemistrynoun
Etymology:
The Use Of Dots In Inorganic Chemistry
Let’s take the example of copper sulfate penta-hydrate: $\ce$. The dot here is used essentially as an expression ofignorance to indicate that, though the parts of themolecule separated by the dot are bonded to one another in some fashion, the exact structural details of that interaction are not fully expressed in the resultingformula.
Using Alfred Werners coordinationtheory that indicatesthat four of the five water molecules are actuallybonded directly to the $\ce$ in the form of a complexaquo ion $\ce$, then the formula is betterexpressedas $\ce$
So, the dot here signifies ignorance of how thesubunits of a formula are structurally related.
Also Check: Kendall Hunt Geometry Answer Key
Potassium In The Periodic Table
In the periodic table, potassium is in Group 1 and Period 4. It is an alkali metal with atomic number 19. The electron configuration of potassium is 4s1. Like other alkali metals, it has a single valence electron that is easily removed, creating a cation. The cationic form of potassium combines with different anions to form salts.
What Is A Chemical Equation
nomenclature
A chemical equation is an expression of a chemical process. For example:
In this equation, AgNO3 is mixed with NaCl. The equation shows that the reactants react through some process to form the products . Since they undergo a chemical process, they are changed fundamentally.
Often chemical equations are written showing the state that each substance is in. The sign means that the compound is a solid. The sign means the substance is a liquid. The sign stands for aqueous in water and means the compound is dissolved in water. Finally, the sign means that the compound is a gas.
Coefficients are used in all chemical equations to show the relative amounts of each substance present. This amount can represent either the relative number of molecules, or the relative number of moles . If no coefficient is shown, a one is assumed.
On some occasions, a variety of information will be written above or below the arrows. This information, such as a value for temperature, show what conditions need to be present for a reaction to occur. For example, in the graphic below, the notation above and below the arrows shows that we need a chemical Fe2O3, a temperature of 1000 degrees C, and a pressure of 500 atmospheres for this reaction to occur.
The graphic below works to capture most of the concepts described above:
You May Like: Why Is Physics Considered To Be The Basic Science
Chemical Properties Of Potassium
Just like other alkali metals, potassium is very reactive. It can react with water violently to produce hydrogen gas. The excessive heat generated by this reaction, together with the H2 as a product, can be explosive. Potassium can also react with air and form oxide layers. When potassium is burned in the air, it can form the orange potassium superoxide in the form of KO2. Potassium can also react rapidly with all the halogens to form potassium halides .
Detection By Taste Buds
Potassium can be detected by taste because it triggers three of the five types of taste sensations, according to concentration. Dilute solutions of potassium ions taste sweet, allowing moderate concentrations in milk and juices, while higher concentrations become increasingly bitter/alkaline, and finally also salty to the taste. The combined bitterness and saltiness of high-potassium solutions makes high-dose potassium supplementation by liquid drinks a palatability challenge.
Don’t Miss: Houghton Mifflin Harcourt Geometry Workbook Answers
Ten Interesting & Fun Facts About Potassium
What Does Q And W Mean In Chemistry
. Correspondingly, what is Q and W?
The first law of thermodynamics is given as U = QW, where U is the change in internal energy of a system, Q is the net heat transfer , and W is the net work done .
Beside above, what is Delta U Q W? Here UDelta UU is the change in internal energy U of the system. Q Q. Q is the net heat transferred into the systemthat is, Q is the sum of all heat transfer into and out of the system. W W. W is the net work done on the system.
Herein, what does Q stand for in chemistry thermodynamics?
Heat in thermodynamicsScientists define heat as thermal energy transferred between two systems at different temperatures that come in contact. Heat is written with the symbol q or Q, and it has units of Joules .
How do you define enthalpy?
Enthalpy is a thermodynamic property of a system. It is the sum of the internal energy added to the product of the pressure and volume of the system. It reflects the capacity to do non-mechanical work and the capacity to release heat. Enthalpy is denoted as H specific enthalpy denoted as h.
Don’t Miss: Elastic Force Physics
Do Equilibrium Constants Have Units
The correct answer is usually they do.The units are decided by the calculation for Keq.Every time you calculate an equilibrium constant, you need to calculate the units for the equilibrium constant too.
For example, let’s look at the gas phase reaction that produces ammonia from hydrogen and nitrogen.The reaction equilibrium is:
N2 + 3H2
The equilibrium constant is:
2Keq = ---------------- PN2 3
If the partial pressures of each gas are measured in atmospheres, the units of the equilibrium constant for this reaction will be:
atm2Units = ---------------- atm . atm3
Effect Of Cooling On The Value Of The Equilibrium Constant K
If our reaction is endothermic, it absorbs energy, so energy can be considered as a reactant in the chemical equilibrium equation:
| reactants |
|---|
We can see that K’ will be a smaller number than K because:
- the value of the numerator has decreased from to
- and the value of the denominator has increased from to
- and a smaller number divided by a larger number results in a smaller number
If a reaction is endothermic, the value of the equilibrium constant decreases when the reaction mixture is cooled.
Endothermic reaction: decreasing temperature, decreases the value of K
If our reaction is exothermic, it releases energy, so energy can be considered as a product in the chemical equilibrium equation:
| reactants |
|---|
We can see that K’ will be a larger number than K because:
- the value of the numerator has increased
- and the value of the denominator has decreased
- and a larger number divided by a smaller number results in a larger number
If a reaction is exothermic, the value of the equilibrium constant increases when the reaction mixture is cooled.
Exothermic reaction: decreasing temperature, increases the value of K
Can you apply this?
Take the exam now!
You May Like: Lesson 1.7 Practice A Geometry Answers