Kinetic Characterization Of L
The activity of L-LDH was measured spectrophotometrically following the NADH oxidation at 340nm at 37°C in a 50mM buffer Na2HPO4 pH 7.4 . The reaction mixture contained 1mM NADH, 50ng of L-lactate dehydrogenase from rabbit muscle and pyruvate was added to start the reaction. For Km determinations, a saturation curve of pyruvate was generated by varying the concentrations from 0 to 3mM at saturating concentrations of NADH . For the saturation curve for NADH, this substrate was varied from 0 to 1mM at saturating concentrations of Pyruvate . For Ki determinations, similar experiments for Km determinations were done in the presence of different concentrations of oxaloacetate . For the Ki determination of oxaloacetate versus pyruvate, the reaction mixture containing NADH, L-LDH and oxaloacetate was incubated for 1min and then the reaction was started by adding pyruvate. All the substrates and inhibitor were prepared fresh and the oxaloacetate stock solution was kept on ice maximum for 2h. The kinetic data was analysed using the software Origin 9.0.
How Do You Identify Variables In An Experiment
An easy way to think of independent and dependent variables is, when youre conducting an experiment, the independent variable is what you change, and the dependent variable is what changes because of that. You can also think of the independent variable as the cause and the dependent variable as the effect.
Enzyme Inhibitors As Metabolic Poisons
-
Many poisons work by inhibiting the action of enzymes involved in Metabolic processes, which disturbs an organism.
-
For example, Potassium Cyanide is an irreversible Inhibitor of the enzyme Cytochrome C Oxidase, which takes part in respiration reactions in cells. If this enzyme is inhibited, ATP cannot be made since Oxygen use is decreased. This means that cells can only respire Anaerobically, leading to a build up of Lactic Acid in the blood. This is potentially fatal.
-
The poison Malonate binds to the Active Site of the enzyme Succinate Dehydrogenase competing with Succinate, which is important in respiration.
You May Like: Renate Blauel 2018
What Does Inhibited Mean Sexually
A sexual inhibition is a conscious or subconscious constraint or curtailment by a person of behavior relating to specific sexual matters or practices, a discussion of sexual matters or viewing certain sexual material. Such inhibitions also tend to decrease with improvements in a persons confidence in their sexuality.
What Happens When We Degrade
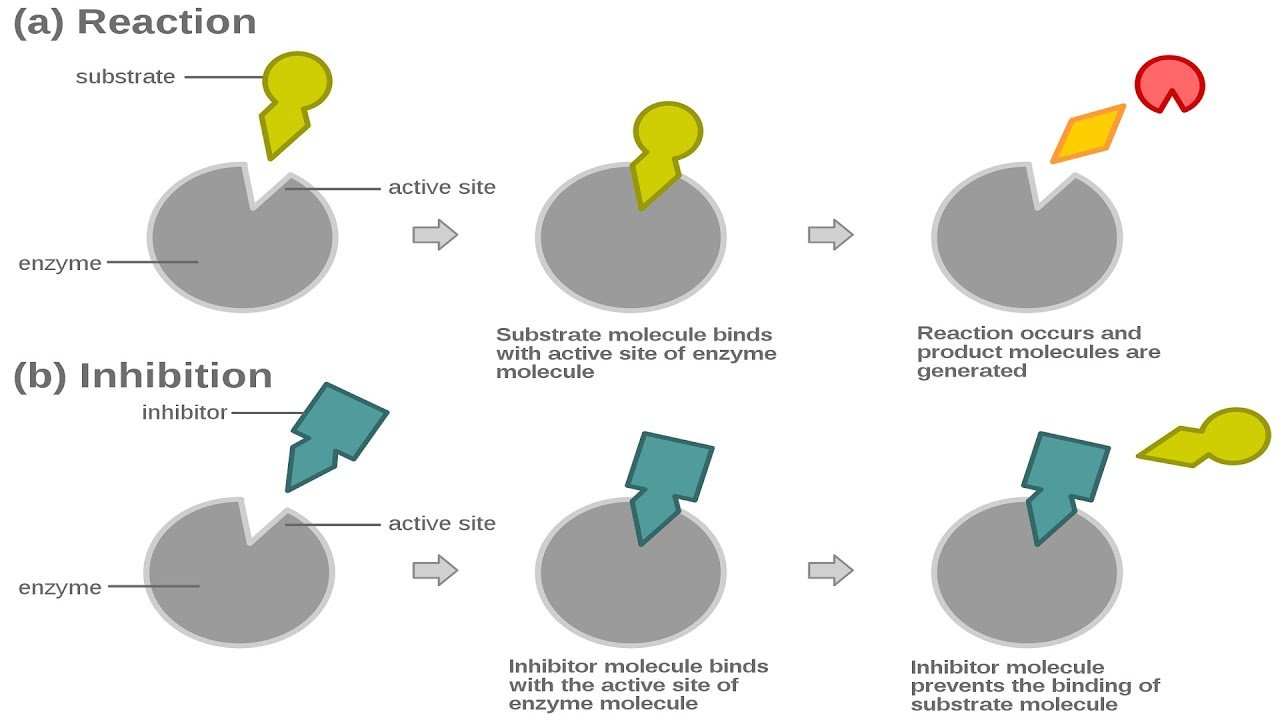
To degrade is defined as to treat someone with disrespect, to lower someones rank, to make something not as good, or to break down or deteriorate. When you talk down to someone and insult him, this is an example of a time when you degrade the person. To lower in quality or value make inferior or less valuable.
Read Also: Holt Mcdougal Geometry Workbook Answers Pdf
Examples Of Reversible Inhibitors
As enzymes have evolved to bind their substrates tightly, and most reversible inhibitors bind in the active site of enzymes, it is unsurprising that some of these inhibitors are strikingly similar in structure to the substrates of their targets. Inhibitors of DHFR are prominent examples. Other example of these substrate mimics are the protease inhibitors, a very successful class of antiretroviral drugs used to treat HIV. The structure of ritonavir, a protease inhibitor based on a peptide and containing three peptide bonds, is shown on the right. As this drug resembles the protein that is the substrate of the HIV protease, it competes with this substrate in the enzyme’s active site.
Enzyme inhibitors are often designed to mimic the transition state or intermediate of an enzyme-catalyzed reaction. This ensures that the inhibitor exploits the transition state stabilising effect of the enzyme, resulting in a better binding affinity than substrate-based designs. An example of such a transition state inhibitor is the antiviral drug oseltamivir this drug mimics the planar nature of the ring oxonium ion in the reaction of the viral enzyme neuraminidase.
Important Compounds And Applications
10.16.7.10.1Pyridopyridazines
A new class of enzyme inhibitors, such as pyridopyridazine 1021 and 1022, have IC50 of 0.2 and 0.6 against p38 map kinase, respectively.91
10.16.7.10.2Pyrido/pyrano/thiopyrano pyrimidines
Compounds containing pyridopyrimidine and pyridopyrimidin-7-ones display a wide range of medicinal and pharmacological activities, including inhibition of p21-activated kinases,477 mitogen-activated protein kinase 4,478 rapidly accelerated fibrosarcoma kinases,192 Wee-1 kinases,479 and hepatitis C virus,480 along with specific treatment of lymphoma by 1023.481 Studies have identified 1024 as a low-nanomolar cell-based dual Akt 1 and 2 inhibitors.482 Interestingly this ring system, as observed in 1025, was identified as a new class of fibroblast growth factor receptor tyrosine kinase inhibitor.483 Pyridopyrimidine 1026 selectively inhibits dihydrofolate reductase with an IC50 of 0.0042 mM for Pneumocystis jirovecii DFHR,484 a causative agent of pneumonia in HIV/AIDS patients.
10.16.7.10.3Pyrido pyrazines
A range of biological activity has been observed for compounds containing pyridopyrazines derivatives, including extracellular signalregulated kinase 2 inhibition by 1041,505 p38R MAP kinase inhibition by 1042 ,506 and transglutaminase 2 inhibition by 1043.507
10.16.7.10.4Pyrido oxazines
Cecilia Pozzi, … Stefano Mangani, in, 2018
Also Check: Geometry Basics Unit 1 Test
Types Of Reversible Inhibitors
Reversible inhibitors attach to enzymes with non-covalent interactions such as hydrogen bonds, hydrophobic interactions and ionic bonds. Multiple weak bonds between the inhibitor and the active site combine to produce strong and specific binding. In contrast to substrates and irreversible inhibitors, reversible inhibitors generally do not undergo chemical reactions when bound to the enzyme and can be easily removed by dilution or dialysis.
There are four kinds of reversible enzyme inhibitors. They are classified according to the effect of varying the concentration of the enzyme’s substrate on the inhibitor.
These types can also be distinguished by the effect of increasing the substrate concentration on the degree of inhibition caused by a given amount of inhibitor. For competitive inhibition the degree of inhibition is reduced by increasing , for noncompetitive inhibition the degree of inhibition is unchanged, and for uncompetitive inhibition the degree of inhibition increases with .
Definition And Mechanisms Of Action
Enzyme activators are chemical compounds that increase a velocity of enzymatic reaction. Their actions are opposite to the effect of enzyme inhibitors. Among activators we can find ions, small organic molecules, as well as peptides, proteins, and lipids.
There are many enzymes that are specifically and directly activated by small inorganic molecules, mainly by cations such as Ca2+ which is a the second messenger . These enzymes usually have special site for Ca2+ binding the binding of Ca2+ with it results in the change of enzyme conformation that increase enzyme activity .
Cations can bind not only with enzyme but also with the substrate increasing its affinity to the enzyme that activate enzyme. For example, magnesium ions interact with ATP or with other nucleotides that are negatively charged molecules, decreasing their charge that provides effective binding of nucleotides in substrate binding site of various enzymes and increasing their activity.
In some cases, activation of enzymes is due to the elimination of enzyme inhibitors. In total this effect looks as enzyme activation. Some cations including heavy metal cations inhibit definite enzymes. Small organic compounds like ethylene glycol-bis-N,N,N,N-tetraacetic acid and ethylenediaminetetraacetic acid that are known as chelating agents bind these inhibitory cations and by this way can eliminate their inhibitory effect.
Also Check: Practice And Homework Lesson 4.5
To Your Health: Penicillin
Chemotherapy is the strategic use of chemicals to destroy infectious microorganisms or cancer cells without causing excessive damage to the other, healthy cells of the host. From bacteria to humans, the metabolic pathways of all living organisms are quite similar, so the search for safe and effective chemotherapeutic agents is a formidable task. Many well-established chemotherapeutic drugs function by inhibiting a critical enzyme in the cells of the invading organism.
An antibiotic is a compound that kills bacteria it may come from a natural source such as molds or be synthesized with a structure analogous to a naturally occurring antibacterial compound. Antibiotics constitute no well-defined class of chemically related substances, but many of them work by effectively inhibiting a variety of enzymes essential to bacterial growth.
Penicillin functions by interfering with the synthesis of cell walls of reproducing bacteria. It does so by inhibiting an enzymetranspeptidasethat catalyzes the last step in bacterial cell-wall biosynthesis. The defective walls cause bacterial cells to burst. Human cells are not affected because they have cell membranes, not cell walls.
Some strains of bacteria become resistant to penicillin through a mutation that allows them to synthesize an enzymepenicillinasethat breaks the antibiotic down . To combat these strains, scientists have synthesized penicillin analogs that are not inactivated by penicillinase.
Enrichment Of Class Of Inhibitors Inhibiting Classes Of Enzymes
Inhibitors were counted for each class of compounds, and inhibitory interactions for each class of enzymes. The phyper function in R was used for Hypergeometric test. P value 0.05 was used as significance threshold. False discovery rate for inhibition of a class of enzymes by a class of inhibitors was calculated as follows. First, inhibitors were randomly assigned to each inhibitor class, and this process was repeated for a hundred times. Then, an enrichment analysis for inhibition of a class of enzymes was performed on randomly assigned inhibitor classes. Finally, if P value of inhibition was better for a random group of inhibitors than the original inhibitor class then the counter was increased. FDR=counter/100.
Don’t Miss: What Is The Meaning Of Abiotic
What Do The The Different Arrowheads Mean In A Cell Signalling Diagram
What do the different arrowheads mean in the figure below? Are there arrows upstream or downstream signalling?
- 3$\begingroup$Hi and welcome to Bio.SE! This is pretty standard notation. The arrowhead is a promotion, and flathead is an inhibition.$\endgroup$Oct 18 ’19 at 17:39
- 1$\begingroup$Hi James! Thanks for the answer! Do you happen to know any resources in general for these notion? and other types of pathway like dotted lines and etc?$\endgroup$Oct 18 ’19 at 17:46
- $\begingroup$i dont know the usage of dotted lines @Tom$\endgroup$ Always ConfusedOct 18 ’19 at 17:49
- $\begingroup$There is an interesting proposal paper by Hiroaki Kitano. However all books should provide a table of all molecular biology notations and conventions but unfortunately these are not yet available.$\endgroup$
Measuring The Dissociation Constants Of A Reversible Inhibitor
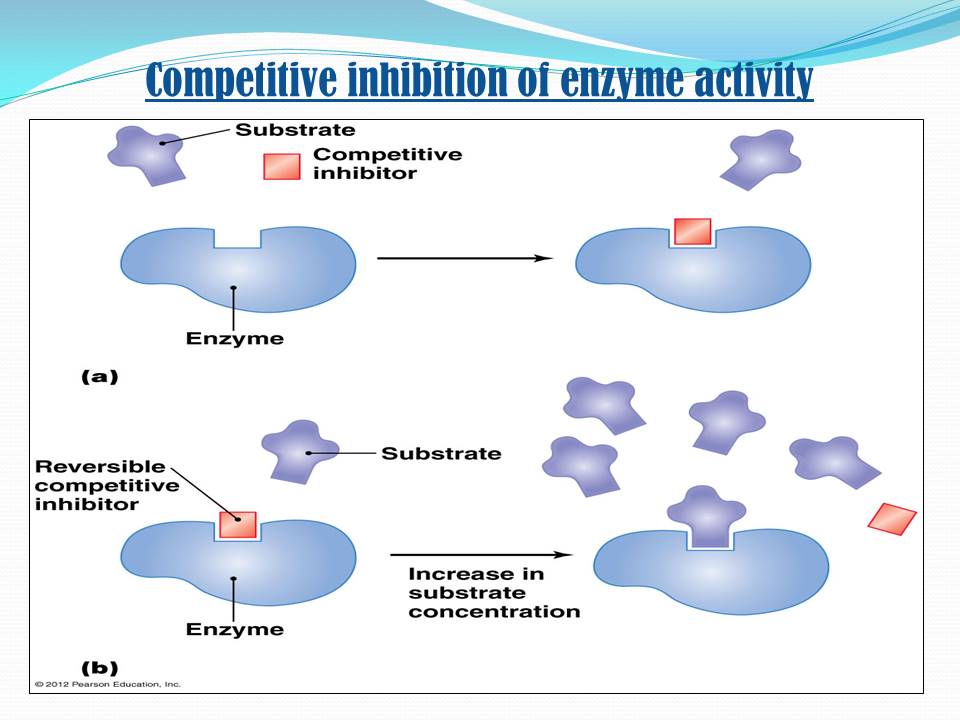
As noted above, an enzyme inhibitor is characterised by its two dissociation constants, Ki and Ki’, to the enzyme and to the enzyme-substrate complex, respectively. The enzyme-inhibitor constant Ki can be measured directly by various methods one extremely accurate method is isothermal titration calorimetry, in which the inhibitor is titrated into a solution of enzyme and the heat released or absorbed is measured. However, the other dissociation constant Ki’ is difficult to measure directly, since the enzyme-substrate complex is short-lived and undergoing a chemical reaction to form the product. Hence, Ki’ is usually measured indirectly, by observing the enzyme activity under various substrate and inhibitor concentrations, and fitting the data to a modified MichaelisMenten equation
- V
- . =1+^}}.}
Thus, in the presence of the inhibitor, the enzyme’s effective Km and Vmax become Km and Vmax, respectively. However, the modified Michaelis-Menten equation assumes that binding of the inhibitor to the enzyme has reached equilibrium, which may be a very slow process for inhibitors with sub-nanomolar dissociation constants. In these cases, it is usually more practical to treat the tight-binding inhibitor as an irreversible inhibitor however, it can still be possible to estimate Ki’ kinetically if Ki is measured independently.
Don’t Miss: Michael Jackson Kids Biological Parents
Definition Of Zone Of Inhibition
The testing for sensitivity of an organism to antimicrobial agents is usually done using agar diffusion or disk diffusion test. The parameters of this test were specified by the scientists W. M. M. Kirby and A. W. Bauer and is also referred to as the Kirby-Bauer antibiotic testing. In this method, antibiotics are impregnated on a certain special type of paper disks and are placed on the surface of agar containing the bacterium of our interest. This results in the diffusion of antimicrobial agent into the surrounding medium.
If the bacterium is sensitive to the particular antibiotic, no growth will be observed. However, as the antibiotic diffuses further, its concentration is reduced. After a certain point, its concentration is so low that it can no longer inhibit the growth of the bacterium. Therefore, there is an area around the disks that will be clear against a dense growth of the bacterium surrounding it, this zone of clearance is defined as the zone of inhibition.
The diameter of the zone of inhibition will determine the effectiveness of the antibiotic the larger the diameter, the greater will be the sensitivity of the bacterium to the antibiotic. The zone sizes are compared to a standardized chart to determine if the bacterium is sensitive, resistant, or shows intermediate sensitivity to that antibiotic.
Antioxidant Peptides Exhibit Acei Activity
Oxidative stress caused by either increased production of reactive oxygen or compromising the biological antioxidant defense system is an issue in the development and progression of CVD.30 Since increased oxidative stress or free radicals cause oxidative damage to a number of intracellular or extracellular molecules, such nucleic acids, proteins, carbohydrates or lipids, or even other smaller physiologically important molecules, they have been implicated in other diseases such as diabetes, cancer, or obesity, conditions that may aggravate the pathogenesis of CVD. Owing to the fact that hypertension is associated with oxidative stress, antioxidant-rich diets reduce the arterial blood pressure in hypertensive subjects and modulate endothelial function.
John T. Welch, in, 2008
You May Like: Elton Johns Parents
How Do Inhibitors Work To Prevent An Enzyme From Functioning Quizlet
-The inhibitor changes the conformation of the enzyme. The substrate can no longer bind, or it may be able to bind but the active site cannot catalyse the reaction, or catalyses it at a slower rate. The activity of an enzyme is reduced if a fixed low concentration of a competitive inhibitor is added.
Types Of Irreversible Inhibition
Irreversible inhibitors usually covalently modify an enzyme, and inhibition can therefore not be reversed. Irreversible inhibitors often contain reactive functional groups such as nitrogen mustards, aldehydes, haloalkanes, alkenes, Michael acceptors, phenyl sulfonates, or fluorophosphonates. These nucleophilic groups react with amino acid side chains to form covalent adducts. The residues modified are those with side chains containing nucleophiles such as hydroxyl or sulfhydryl groups these include the amino acids serine , cysteine, threonine, or tyrosine.
Irreversible inhibition is different from irreversible enzyme inactivation. Irreversible inhibitors are generally specific for one class of enzyme and do not inactivate all proteins they do not function by destroying protein structure but by specifically altering the active site of their target. For example, extremes of pH or temperature usually cause denaturation of all protein structure, but this is a non-specific effect. Similarly, some non-specific chemical treatments destroy protein structure: for example, heating in concentrated hydrochloric acid will hydrolyse the peptide bonds holding proteins together, releasing free amino acids.
Don’t Miss: Chapter 4 Test Form 1 Algebra 2 Answers
What Is The Role Of Competitive Inhibitor
The competitive inhibitor is an enzyme inhibitor, a molecule that binds to an active site of the enzyme and decreases its activity by preventing the binding of substrate to the active site of an enzyme. Because of the presence of the inhibitor, fewer active sites are available to act on the substrate.
Selection Against Unwanted Inhibition
To examine whether the selection of unwanted inhibitions within compartments affected competitive and allosteric inhibition modes differentially, we used the set of gold standard examples for which the type of inhibition is known with the greatest reliability . We calculated a ratio that compares the occurrence of competitive versus allosteric inhibition in a compartment-dependent manner. A ratio of substrate-inhibitor pairs within the same compartment, versus the S-I pair within the different compartment, is more pronounced for competitive inhibition compared to allosteric inhibition .
Recommended Reading: Geometry Dash 1 20
What Is Inhibition In Biology
4.8/5biologyinhibitbiological inhibitorinhibitorbiologicalanswer here
Inhibit comes from the Latin inhibitus, meaning “to hold in”, “to restrain”, or “to keep”. In biology, there are various molecules whose function is to inhibit. In biology, an inhibiting molecule controls, prevents, restrains, arrests, or regulates, as in “to inhibit an action”.
One may also ask, what are the three types of enzyme inhibition? We will discuss four types of enzyme inhibition competitive, non- competitive, uncompetitive, and suicide. Of these, the first three types are reversible.
Also to know is, what does an inhibitor do?
Enzyme inhibitors are molecules or compounds that bind to enzymes and result in a decrease in their activity. An inhibitor can bind to an enzyme and stop a substrate from entering the enzyme’s active site and/or prevent the enzyme from catalyzing a chemical reaction. There are two categories of inhibitors.
What is a non competitive inhibitor in biology?
Non–competitive inhibition is a type of enzyme inhibition where the inhibitor reduces the activity of the enzyme and binds equally well to the enzyme whether or not it has already bound the substrate.
How To Measure The Zone Of Inhibition
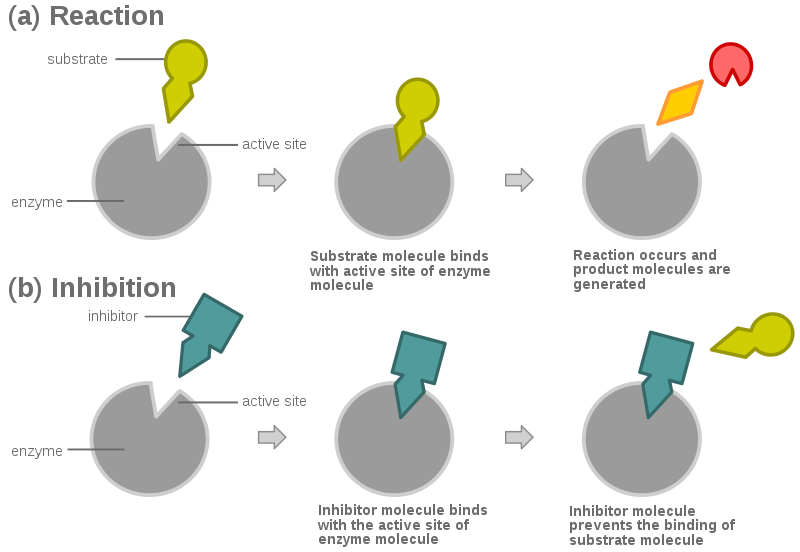
The zone of inhibition is measured using a ruler, a pair of calipers, or with the help of a template. Its size is measured in millimeters and usually rounded off to the closest millimeter. The diameter of the disk is also included.
These measurements are done by the naked eye without the help of any instrument. The measurement of the diameter is made from the back of the plate. These plates are held against a dark background.
These plates should be viewed directly from above in order to minimize the errors while measurement.
In case of organisms showing swarming motility, ignore the veil of the organisms that might swarm into the zone of inhibition.
In case there is no zone of inhibition around the disk, consider the zone of inhibition zero.
On blood agar, the zone of inhibition is measured from the upper surface of the agar and is done by removing the lid of the Petri plate.
You May Like: Mcdougal Littell Geometry Chapter 7 Test Answers