Example : Using Displacement Of Water To Determine Density
This PhET simulation illustrates another way to determine density, using displacement of water. Determine the density of the red and yellow blocks.
When you open the density simulation and select Same Mass, you can choose from several 5.00-kg colored blocks that you can drop into a tank containing 100.00 L water. The yellow block floats , and the water level rises to 105.00 L. While floating, the yellow block displaces 5.00 L water, an amount equal to the weight of the block. The red block sinks , and the water level rises to 101.25 L.
The red block therefore displaces 1.25 L water, an amount equal to the volume of the block. The density of the red block is:
\large\text=\frac}}=\frac}}=4.00 kg/L
Note that since the yellow block is not completely submerged, you cannot determine its density from this information. But if you hold the yellow block on the bottom of the tank, the water level rises to 110.00 L, which means that it now displaces 10.00 L water, and its density can be found:
\large\text=\frac}}=\frac}=\text
Check Your Learning
Remove all of the blocks from the water and add the green block to the tank of water, placing it approximately in the middle of the tank. Determine the density of the green block.
\large 7.88\cancel}}\times\frac}}}}=21\text
where we have limited our answer to two significant figures.
Applications Of Density In Real Life
Many applications of density are there in our real-life like a few examples are in pipe design, shipbuilding, helium balloons, weight distribution in the airplane, and the fact that ice floats on water.
- The knowledge of the densities of two substances helps you in separation techniques. For example, separation of oil from water. Leakage of an oil tank in the ocean then oil drops start to float on the water due to their less density in the water.
- Another well-known application of density is determining whether an object will float on water or not. The floating of ships and diving of submarines are due to their density difference.
How Do You Calculate Density
Always keep an eye on your units: it’s standard to use g or kg per cm³ or m³, so use our unit converters if your measurements are in different units.
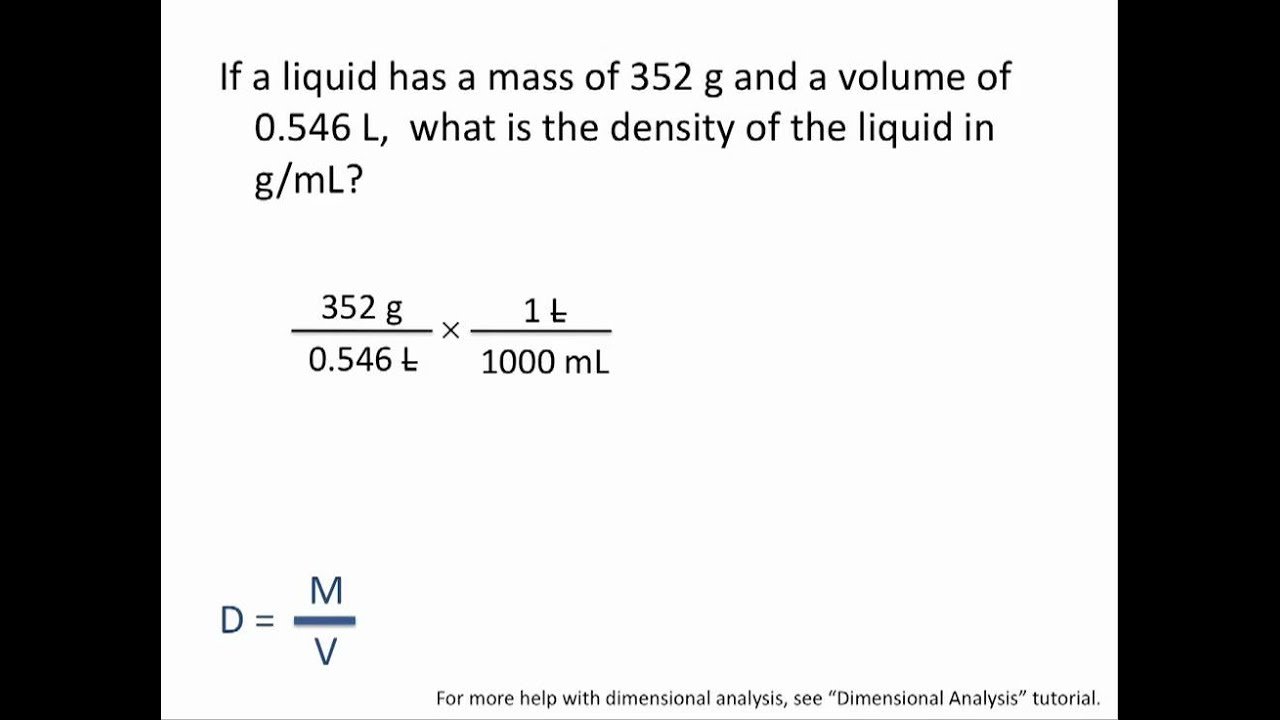
To calculate density, you divide the mass by the volume:
Density = Mass ÷ Volume
Density is often written in mathematics as the symbol p or D. Mass can be written as m, and volume can be written as V. So if you want to be fancy, the formula looks like this:
p = m/V
So, if you have a kilogram of cheese with the volume of 0.0002m³, then not only are you in for some weird and wonderful dreams, but you also have cheese with a density of 5000kg/m³ .
Try our density calculator to make it all a piece of cake. Hopefully even better than Auntie Mabel’s lemon sponge…
Also Check: What Happened To Beth Child Of Rage
How Do You Find Density Without Mass And Volume
A simple method based on the moment of forces and Archimedes principle is described for finding density without measuring the mass and volume of an object. The method involves balancing two unknown objects of masses M1 and M2 on each side of a pivot on a metre rule and measuring their corresponding moment arms.
How To Find Density In Chemistry 2022
How To Find Density In Chemistry. The formula for density is d = m/v, where d is density, m is mass, and v is volume. A dense object weighs more than a less dense object that is the same size.
For example, you measure a quantity of pure water in a graduated cylinder and see it comes to 11.5 ml. You’ll find the answers to each question at the bottom of the page.
Don’t Miss: All Geometry Dash Vault Codes
Have Students Consider Whether The Density Of A Large Piece Of A Solid Substance Is The Same As The Density Of A Smaller Piece
Give students time to calculate the density of each of the three samples drawn on their activity sheet and answer the related questions.
Ask students:
- The density of a liquid is the same no matter what the size of the sample. Could this be true for solids, too? Calculate the density of each of the three samples to find out.
- Yes. The density of a solid substance is the same no matter how big or small the sample.
- Sample A has a mass of 200 g. What is the density of Sample A?
- If you cut Sample A in half and looked at only one half, you would have Sample B. What is the density of Sample B?
- If students do not know what the mass is, tell them that it is half the mass of Sample A. Because Sample A was 200 g, Sample B is one half the volume and therefore one half the mass .
-
- D = m/v
Density Of Distilled Water: Page 15 Part Iic
1. Corrected Mass and Volume After Each Addition of Water
We don’t really care what the beaker you used weighs, and we don’t care to what level you filled the buret before you started taking water samples from it: we just need the mass of each water sample and its volume. Remember that we for each set of data you recorded in Part C on Page 13, we want the total cumulative mass and volume after each addition of water . To calculate the volume after each addition, subtract the initial reading of the buret before any water was dispensed from the current buret reading for a particular sample.
2. Graph
It is essential that you follow the graphing instructions given in the Appendix at the back of the lab manual for making your graph. Graphs count a lot in lab and you will receive major penalties if you do not follow the instructions provided. There is also a sample graph for this experiment posted in the laboratory.
You May Like: Geometry Warm Ups
Calculations To Find Density
To find density, divide the measured mass by the measured volume .
Density Formula Examples: Solids
If a cube of a material measuring 1 centimeter on each side has a mass of 7.90 grams, the density calculation becomes
The material is most likely iron.
The mass of an irregularly shaped object is measured as 211.4 grams. The volume of water displaced equals 20 milliliters. Since one milliliter of water occupies one cubic centimeter of volume, the object’s volume equals 20 cubic centimeters. Completing the formula shows
The material is most likely silver.
Density Formula Examples: Liquids
A liquid with a volume of 50 milliliters has a mass of 63 grams . So
The liquid is likely to be glycerine.
A liquid with a measured mass of 338.75 grams occupies a volume of 25 milliliters. Completing the density formula shows
The liquid is probably mercury.
What Is Density Definition
Density is the measure of how much stuff is in a given amount of space. For example, a block of the heavier element lead will be denser than the softer, lighter element gold . A block of Styrofoam is less dense than a brick. It is defined as mass per unit volume.
You can think of it, as how tightly or loosely packed a substance is, or how compact it is. Solids are generally more dense than liquids, and liquids more dense than gases, but there are many exceptions.
Also Check: Lewis Dot Structure For Ccl4
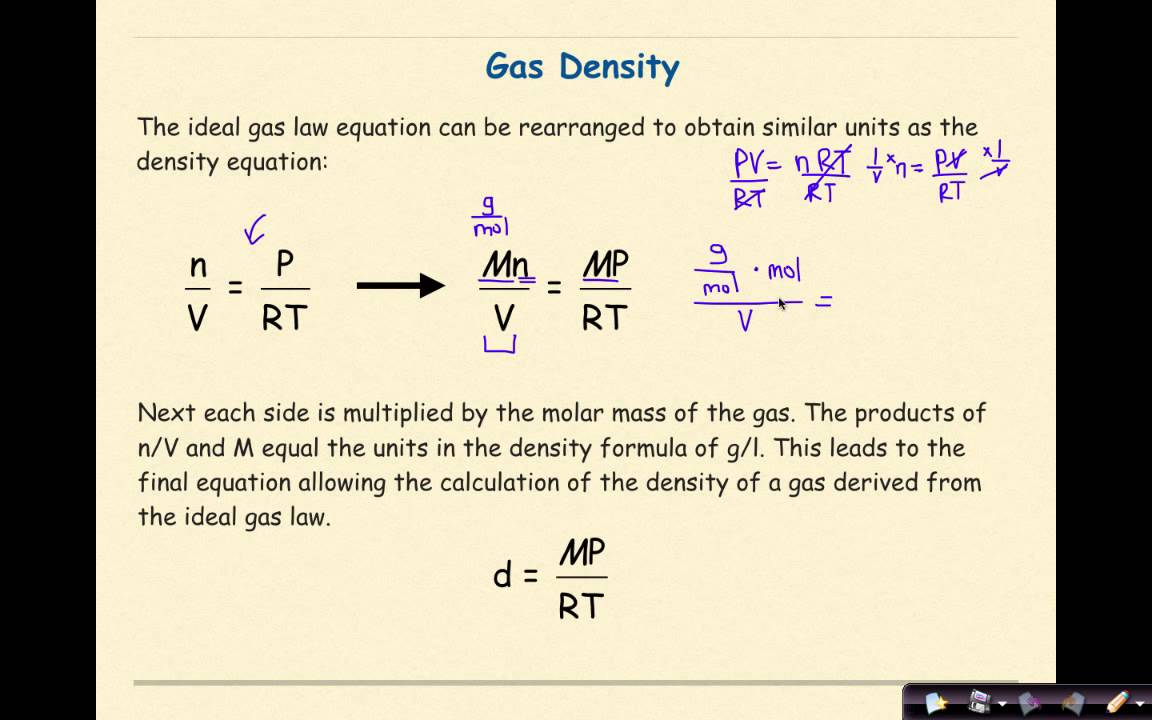
Pressure Vs Density Graph For Ideal Gas
The pressure versus limiting density graph of an ideal gas is shown below the figure,
The direct use of the limiting density formula causes some difficulty since, at P0, 0 and not possible to determine experimentally. Therefore, the above graphical measurement has taken advantage of the calculation of molar mass.
Problem: At 0° temperature, the density of gaseous non-metallic oxide at 2 atm pressure is the same as that of oxygen at 5 atm. Find the molecular weight of the nonmetallic substance.
Solution: From the density formula, M = MO2 × = 32 × = 32 = 80 g/mol.
Determining The Ratio Between Mass And Volume
- M.S., Mathematics Education, Indiana University
- B.A., Physics, Wabash College
A material’s density is defined as its mass per unit volume. Put another way, density is the ratio between mass and volume or mass per unit volume. It is a measure of how much “stuff” an object has in a unit volume . Density is essentially a measurement of how tightly matter is crammed together. The principle of density was discovered by the Greek scientist Archimedes, and it is easy to calculate if you know the formula and understand its related units.
Read Also: Redken Chemistry Shot Vs Olaplex
Using Water As A Density Comparison
When an object is placed in water, the objects relative density determines whether it floats or sinks. If the object has a lower density than water, it will float to the top of the water. An object with a higher density will sink. For example, cork has a density of 240 kg/m3, so it will float. Air has a density of approximately 1.2 kg/m3, so it rises immediately to the top of a water column. The metals sodium and potassium will both float on water, while lead will sink.
Density: A Story of Archimedes and the Gold Crown
Liquids tend to form layers when added to water. The sugar alcohol glycerol will sink into the water and form a separate layer until it is thoroughly mixed . Vegetable oil will float on water, and no matter how vigorously mixed, will always return as a layer on the water surface .
The Variable Density Of Water
Water itself is a complicated and unique molecule. Even if the pressure is consistent, waters density will change based on the temperature. Recall that the three basic forms of matter are solid, liquid and gas . As a rule of thumb, almost all materials are more dense in their solid or crystalline form than in their liquid form place the solid form of almost any material on the surface of its liquid form, and it will sink. Water, on the other hand, does something very special: ice floats on liquid water.
Look carefully at the relationship between waters temperature and its density. Beginning at 100 °C, the density of water steadily increases, as far as 4 °C. At that point, the density trend reverses. At 0 °C, water freezes to ice and floats.
The density of water at constant pressure
The implications of this simple fact are enormous: when a lake freezes, ice crusts at the surface and insulates the liquid below from freezing, while at the same time allowing the colder water to sink to the bottom. If ice did not float, it would sink to the bottom, allowing more ice to form and sink, until the lake froze solid! Scuba divers and swimmers often encounter these water temperature gradients, and they might even encounter a water layer at the very bottom of a lake with a temperature of approximately 4 °C. Thats just about as cold as the lake will get at the bottom as soon as the water gets colder, the liquid water becomes less dense and rises.
Layers of water in a winter lake
Recommended Reading: Similar Math Definition
How To Find Density
In studying density, it can be helpful to work a sample problem using the formula for density, as mentioned in the previous section. Recall that though density is indeed mass divided by volume, it is often measured in units of grams per cubic centimeter because grams represent a standard weight, while cubic centimeters represent the volume of the object.
For this problem, take a brick of salt measuring 10.0 cm x 10.0 cm x 2.0 cm, which weighs 433 grams. To find the density, use the formula, which helps you determine the amount of mass per unit volume, or:
= m / v
In this example, you have the dimensions of the object, so you have to calculate the volume. The formula for volume depends on the shape of the object, but it’s a simple calculation for a box:
v = length x width x thicknessv = 10.0 cm x 10.0 cm x 2.0 cmv = 200.0 cm3
Now that you have the mass and volume, calculate the density, as follows:
= m / v
Thus, the density of the salt brick is 2.165 g/ cm3.
Key Takeaways: How To Calculate Density
- Density is how much matter is contained within a volume. A dense object weighs more than a less dense object that is the same size. An object less dense than water will float on it one with greater density will sink.
- The density equation is density equals mass per unit volume or D = M / V.
- The key to solving for density is to report the proper mass and volume units. If you are asked to give density in different units from the mass and volume, you will need to convert them.
Question 1: What is the density of a cube of sugar weighing 11.2 grams measuring 2 cm on a side?
Step 1:Find the mass and volume of the sugar cube.
Mass = 11.2 gramsVolume = cube with 2 cm sides.
Volume of a cube = 3Volume = 3
Step 2: Plug your variables into the density formula.
density = mass/volumedensity = 1.4 grams/cm3
Answer 1: The sugar cube has a density of 1.4 grams/cm3.
Question 2: A solution of water and salt contains 25 grams of salt in 250 mL of water. What is the density of the salt water?
Step 1: Find the mass and volume of the salt water.
This time, there are two masses. The mass of the salt and the mass of the water are both needed to find the mass of the salt water. The mass of the salt is given, but the only the volume of water is given. We’ve also been given the density of water, so we can calculate the mass of the water.
densitywater= masswater/volumewater
Answer 2: The salt water has a density of 1.1 grams/mL.
Recommended Reading: Beth Thomas Story
Density And Density Problems
- Page ID
- 54943
Learning Objectives
- To be introduced to the concepts of Density and Percent Composition as important properties of matter.
Density and percent composition are important properties in chemistry. Each have basic components as well as broad applications. Components of density are: mass and volume, both of which can be more confusing than at first glance. An application of the concept of density is determining the volume of an irregular shape using a known mass and density. Determining Percent Composition requires knowing the mass of entire object or molecule and the mass of its components.
Example : Using Density As A Conversion Factor
What is the mass of 44.6 mL of mercury? Mercury has a density of 13.6 g/mL.
\large 44.6\cancel\times\frac}}}=607 \text
Check Your Learning
What is the mass of 25.0 cm3 of iron? Density of iron can be found in Table 1.
Density can also be used as a conversion factor to convert mass to volumebut care must be taken. We have already demonstrated that the number that goes with density normally goes in the numerator when density is written as a fraction. Take the density of gold, for example:
\large \text =19.3\text=\frac}}}
That is, the density value tells us that we have 19.3 grams for every 1 milliliter of volume, and the 1 is an exact number. When we want to use density to convert from mass to volume, the numerator and denominator of density need to be switched. For example, if we want to know the volume of 45.9 g of gold, we would set up the conversion as follows:
\large 45.9\cancel\times\frac}}}=2.38\text
Note how the mass units cancel, leaving the volume unit, which is what were looking for.
Read Also: Percent Difference In Physics
Example : Calculation Of Density
Goldin bricks, bars, and coinshas been a form of currency for centuries. In order to swindle people into paying for a brick of gold without actually investing in a brick of gold, people have considered filling the centers of hollow gold bricks with lead to fool buyers into thinking that the entire brick is gold. It does not work: Lead is a dense substance, but its density is not as great as that of gold, 19.3 g/cm3. What is the density of lead if a cube of lead has an edge length of 2.00 cm and a mass of 90.7 g?
The density of a substance can be calculated by dividing its mass by its volume. The volume of a cube is calculated by cubing the edge length.
\large\text=2.00\text\times 2.00\text\times 2.00\text=}^
\large\text=\frac}}=\frac}}^}=\frac}}^}=}^
Check Your Learning
Density Calculations Worksheet Answers Fresh Stoichiometry
Once you’ve measured the mass and volume of an object, you find density with a simple calculation. Whether students weigh 100, 50, 25 ml or any other amount, the density of water will always be 1 g/cm 3.
How to calculate the mass of an object using water. The formula for density is d = m/v, where d is density, m is mass, and v is volume.
How to use electronegativity to determine electron density. A dense object weighs more than a less dense object that is the same size.
How to calculate the mass of excess reactant left over in. For example, you measure a quantity of pure water in a graduated cylinder and see it comes to 11.5 ml.
Density worksheet with answers calculate density. You’ll find the answers to each question at the bottom of the page.
5 memorable chemistry gas laws worksheet answers di 2020. Once you’ve measured the mass and volume of an object, you find density with a simple calculation.
How to calculate the height of a cylinder using the. Whether students weigh 100, 50, 25 ml or any other amount, the density of water will always be 1 g/cm 3.
Recommended Reading: Elastic Force Equation