Measurement And Use Of Electron Affinity
This property is used to measure atoms and molecules in the gaseous state only, since in a solid or liquid state their energy levels would be changed by contact with other atoms or molecules.
A list of the electron affinities was used by Robert S. Mulliken to develop an electronegativity scale for atoms, equal to the average of the electrons affinity and ionization potential. Other theoretical concepts that use electron affinity include electronic chemical potential and chemical hardness. Another example, a molecule or atom that has a more positive value of electron affinity than another is often called an electron acceptor and the less positive an electron donor. Together they may undergo charge-transfer reactions.
Reaction Scheme And Kinetics Of Propane Oxidation On Mn Oxides
The activation energies for propane oxidation on Mn3O4 and Mn2O3 were measured to be in the range 19-20 kcal/mol. They fall within the range observed for propane combustion on other catalysts and show that experiments are performed under chemical kinetic control. The reaction was found to be first order with respect to propane. Kinetic measures can be interpreted assuming that the above activation energies are related to the first C-H bond breaking and that the reaction path splits into two ways. A common intermediate species has been identified to be a 2-propoxide species that can convert either to propene or to CO2 via adsorbed acetone. Propene over-oxidation can also give rise to CO2 and to CO . The predominant propane combustion way is in defect oxygen through propene and in excess oxygen through adsorbed acetone.
Scheme I. Proposed propane oxidation pathway.
Joseph J. Gajewski, in, 2004
Periodic Trends In Electron Affinity
Although Eea varies greatly across the periodic table, some patterns emerge. Generally, nonmetals have more positive Eea than metals. Atoms, such as Group 7 elements, whose anions are more stable than neutral atoms have a higher Eea. The electron affinities of the noble gases have not been conclusively measured, so they may or may not have slightly negative values. Chlorine has the highest Eea while mercury has the lowest.
Eea generally increases across a period in the periodic table, due to the filling of the valence shell of the atom. For instance, within the same period, a Group-17 atom releases more energy than a Group-1 atom upon gaining an electron because the added electron creates a filled valence shell and therefore is more stable.
A trend of decreasing Eea down the groups in the periodic table would be expected, since the additional electron is entering an orbital farther away from the nucleus. Since this electron is farther away, it should be less attracted to the nucleus and release less energy when added. However, this trend applies only to Group-1 atoms. Electron affinity follows the trend of electronegativity: fluorine has a higher electron affinity than oxygen , and so on.
The trends noted here are very similar to those in ionization energy and change for similar reasons.
Electron affinities in the periodic table
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
You May Like: Geometry Dash Steam Hack
Activation Energy In A 2d Potential Energy Surface
Activation energy can be represented in 2D Potential Energy Surfaces , where the relation between the geometry of the reactants and the energy involved is represented as a topographic map.
In the following graphic there is a representation of a reaction between hydrogen in the gas phase and a metal: tungsten. The potential energy is obtained with PES calculations and consistent with the position of H from the NEB method calculations. A 2-dimensional interpolation with the spline method can be used to evaluate the potential energy at these positions. Products and reactants can be found in the blue surface, however the red surface corresponds to the steady-state approximation.
The depics correspond to the trajectories. The bluer the surface, the stronger the hydrogen bonds, so blue colors represent minima energy and red colors are maxima. Tungstenâs PES is symmetric, and has a dip at the bridge site, this dip corresponds to the change of color in the center of the depic.
The bluer the surface between the energy minima, the lower the energy barriers, and therefore the more easily hydrogen travels along the surfaces.
Presence Or Absence Of A Catalyst
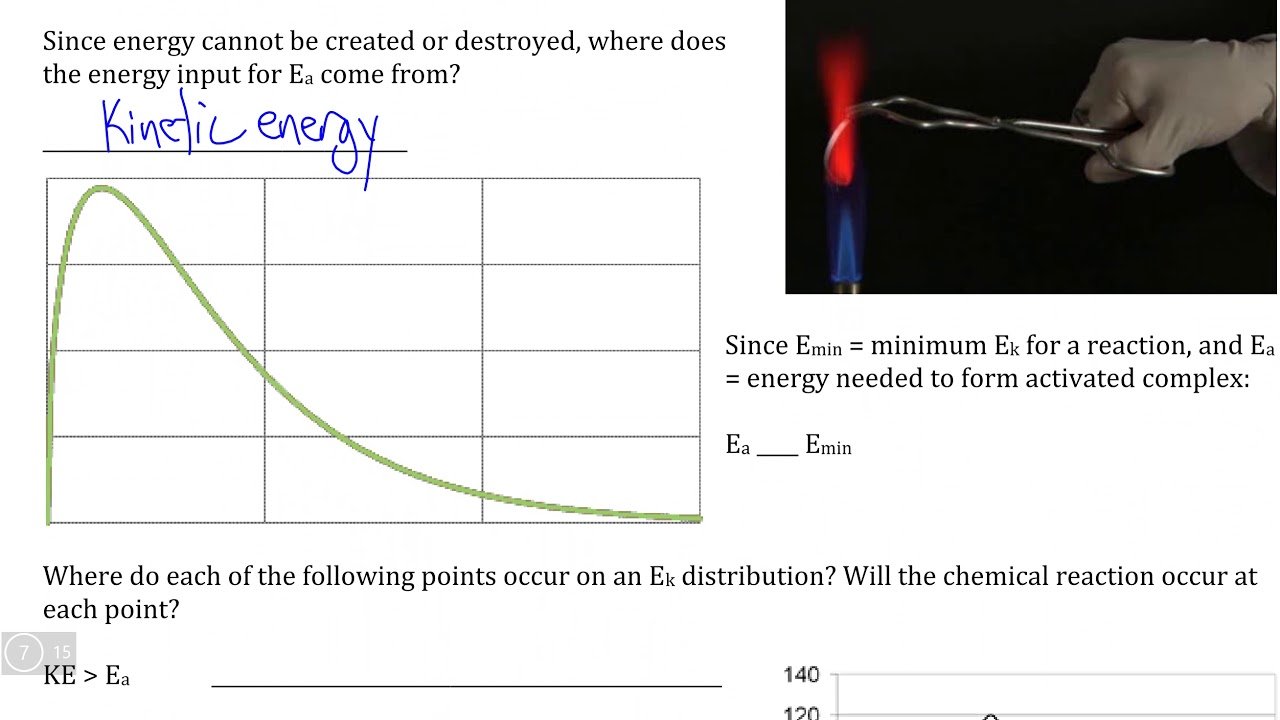
Catalysts are substances that increase reaction rate by lowering the activation energy needed for the reaction to occur. A catalyst is not destroyed or changed during a reaction, so it can be used again. For example, at ordinary conditions, H2 and O2 do not combine. However, they do combine in the presence of a small quantity of platinum, which acts as a catalyst, and the reaction then occurs rapidly.
You May Like: Ccl4 Dot Structure
What Is Fifa Ultimate Team
FIFA Ultimate Team is a game mode in FIFA 21 where you can combine players of different clubs and nationalities to create the perfect XI.
In Ultimate Team, you can create squads of super players, putting Lionel Messi and Cristiano Ronaldo in the same team or build a Premier League XI to take on the world – the possibilities are endless.
There are some restrictions to squad building, meaning you cannot just put any 11 players into a side and hope it works. Each team has a chemistry rating which is calculated from the players in the squad, who need to have some sort of connection to their team-mates to play well.
FIFA Ultimate Team has both offline and online game modes, offering a variety of rewards depending on your skill level and your success in weekly competitions.
What Gets Stored In A Cookie
This site stores nothing other than an automatically generated session ID in the cookie no other information is captured.
In general, only the information that you provide, or the choices you make while visiting a web site, can be stored in a cookie. For example, the site cannot determine your email name unless you choose to type it. Allowing a website to create a cookie does not give that or any other site access to the rest of your computer, and only the site that created the cookie can read it.
Also Check: Founded Behaviorism
Ea Reveals How Chemistry Works In Fifa 19 Ultimate Team
After the recent outcry from the FIFA community over the lack of communication between FIFA developers and the community themselves, EA started to publish Pitch Notes. This is EAs way of communicating with their players, as well as becoming more transparent as a publisher. The FIFA community has been looking for transparency and communication ever since games such as PUBG and Fortnite have been transparent with their communities in terms of updates.
EA recently published their second iteration of Pitch Notes. This specific post was about Chemistry in Ultimate Team. Chemistry is a feature that determines a players performance. If they are linked with players that they are not familiar with , then they will perform worse. In addition to this, EA recently introduced chemistry styles that determine which statistics get a boost for each player. Certain chemistry styles can be applied depending on your style of play.
In FIFA 18, EA introduced a new way of using chemistry styles. Data miners were able to establish the exact amount of increase when it came to applying chemistry styles to an item. This meant that some players could get significant statistic boosts. It was unclear whether this was true for some time. However, EA has finally published a full article explaining and proving the boosts. You can read the official article here.
Ea Meaning In Chemistry
Please also find EA meaning for Chemistry in other sources.
- And finally again and again search .
Recommended Reading: Eoc Fsa Warm Ups Algebra 1 Answers
Electron Affinities Of The Elements
Although Eea varies greatly across the periodic table, some patterns emerge. Generally, nonmetals have more positive Eea than metals. Atoms whose anions are more stable than neutral atoms have a greater Eea. Chlorine most strongly attracts extra electrons neon most weakly attracts an extra electron. The electron affinities of the noble gases have not been conclusively measured, so they may or may not have slightly negative values.
Eea generally increases across a period in the periodic table prior to reaching group 18. This is caused by the filling of the valence shell of the atom a group 17 atom releases more energy than a group 1 atom on gaining an electron because it obtains a filled valence shell and therefore is more stable. In group 18, the valence shell is full, meaning that added electrons are unstable, tending to be ejected very quickly.
Counterintuitively, Eea does not decrease when progressing down the rows of the periodic table, as can be clearly seen in the group 2 data. Thus, electron affinity follows the same “left-right” trend as electronegativity, but not the “up-down” trend.
The following data are quoted in kJ/mol.
How Important Is Chemistry
Hi i’m coming back to fifa after not playing since fifa17 and I was wondering how much chemistry affects player stats since I heard its less important?
I remember it used to be a big deal so wondering if its still the same
Thanks for the help
100 Chem for your team is a must. Individual player chem aim for the highest but 7 is fine too.
do players with 7 chemistry have worse stats?
probably the most important thing if you want your passes to be accurate and player positioning correct. if you had low chem your passes would all be misplaced, players would be all over the place and make runs at the wrong time. its fine if chem is 90+ but below that it starts getting dodgy.
with individual chemistry, if your chemistry is 100 then they should be fine even if a players chemistry says 7 or 8. player may misplace a pass but it’ll be on rare occasion.
Also Check: Geometry Basics Segment Addition Postulate Answer Key
How Do You Find A And Ea Using The Arrhenius Equation
The Arrhenius equation is given by #K=Ae^-)# Use this equation to find out the desired quantity.
Explanation:
The Arrhenius equation is given by #K=Ae^-)# 3. R is Universal Gas Constant4. T is Temperature 5. K is Rate Constant
Before starting of I would like to invoke the old rule of mathematics which states “In order to find the value of all variable the number of equations is equal to the no. of unknowns.”
I am also assuming you know about Chemical Kinetics.The pre-exponential factor and are different for different reacting species under different conditions.
So suppose you don’t know one of the equations say The #E_a# , assume it’s value is x and substitute in the equation.
All the quantities other than R and #E_a# Chose the appropriate value of R based on the table in the link.
Solving for
First Electron Affinity Decreases Down The Group
Moving down a group, the number of energy shells also increases with the increase of protons and electrons. So because of shielding effects of electrons in the increased inner shells, the affinity to an electron of the nucleus is reduced down the group.
Fluorine is an exception
According to the above discussion, fluorine should have more electron affinity than chlorine. But the electron affinity of fluorine is less than chlorine. As fluorine is the smallest atom in this group, entering an electron to it results a greater repulsion because of the existence of electron cloud in the small shell. As a result, electron affinity decreases in fluorine.
| F |
Recommended Reading: Eoc Fsa Warm Ups Algebra 1 Answers
Nature Of The Reactants
Substances differ markedly in the rates at which they undergo chemical change. The differences in reactivity between reactions may be attributed to the different structures of the materials involved for example, whether the substances are in solution or in the solid state matters. Another factor has to do with the relative bond strengths within the molecules of the reactants. For example, a reaction between molecules with atoms that are bonded by strong covalent bonds will take place at a slower rate than would a reaction between molecules with atoms that are bonded by weak covalent bonds. This is due to the fact that it takes more energy to break the bonds of the strongly bonded molecules.
What Is Chemistry In Fifa Ultimate Team
Each player in your starting XI has a chemistry rating which helps determine your team’s overall chemistry rating, up to a maximum of 100. Each player has a maximum chemistry score of 10, meaning you do not need all players to have individual chemistry of 10 to reach an overall score of 100.
A player’s chemistry rating is decided by a few factors: their position, the players around them and their manager.
Players who are played out of position will lose chemistry points, but you can buy position change cards on the transfer market to help turn a defensive midfielder into an attacking midfielder or a right midfielder into a right winger, for example.
Chemistry will be boosted if a player is adjacent to players who share the same club, league or nationality as them. Perfect chemistry comes from playing beside someone who shares the same club and nation – such as German duo Leon Goretzka and Joshua Kimmich in the Bayern Munich midfield.
Each team needs a manager as well. Any player who shares the same league or nationality as their manager will receive a chemistry boost of +1.
Read Also: Geometry Segment Addition Postulate Worksheet
Plotting The Arrhenius Equation In Non
The Arrhenius equation can be written in a non-exponential form, which is often more convenient to use and to interpret graphically. Taking the natural logarithms of both sides and separating the exponential and pre-exponential terms yields: \text=\text-\frac_}}}
Note that this equation is of the form \text=\text+\text, and creating a plot of ln versus 1/T will produce a straight line with the slope Ea/R.
Plot of ln versus 1/T for the decomposition of nitrogen dioxide: The slope of the line is equal to -Ea/R.
This affords a simple way of determining the activation energy from values of k observed at different temperatures. We can plot ln versus 1/T, and simply determine the slope to solve for Ea.
Activation Energy And Temperature
When two billiard balls collide, they simply bounce off of one other. This is also the most likely outcome when two molecules, A and B, come into contact: they bounce off one another, completely unchanged and unaffected. In order for a collision to be successful by resulting in a chemical reaction, A and B must collide with sufficient energy to break chemical bonds. This is because in any chemical reaction, chemical bonds in the reactants are broken, and new bonds in the products are formed. Therefore, in order to effectively initiate a reaction, the reactants must be moving fast enough so that they collide with sufficient force for bonds to break. This minimum energy with which molecules must be moving in order for a collision to result in a chemical reaction is known as the activation energy.
As we know from the kinetic theory of gases, the kinetic energy of a gas is directly proportional to temperature. As temperature increases, molecules gain energy and move faster and faster. Therefore, the greater the temperature, the higher the probability that molecules will be moving with the necessary activation energy for a reaction to occur upon collision.
You May Like: Eoc Fsa Warm Ups Algebra 1 Answers
What Is Division Rivals
Division Rivals is the main weekly online game mode in FIFA 21, allowing you to earn promotion through divisions, face tougher opponents and win better rewards.
Every game you play each week will earn rivals points which calculate your overall rank for the week and also determine whether you can be promoted or relegation from your division.
Depending on your weekly rank, you can choose from three reward options on Thursdays, taking coins, tradeable packs or untradeable packs as your prize.
Each Division Rivals game also earns Weekend League points to help qualify for FUT Champions. Once you get to 2000 points, you can enter the Weekend League and these points can be used at any time, not just the weekend after you reach 2000 points.
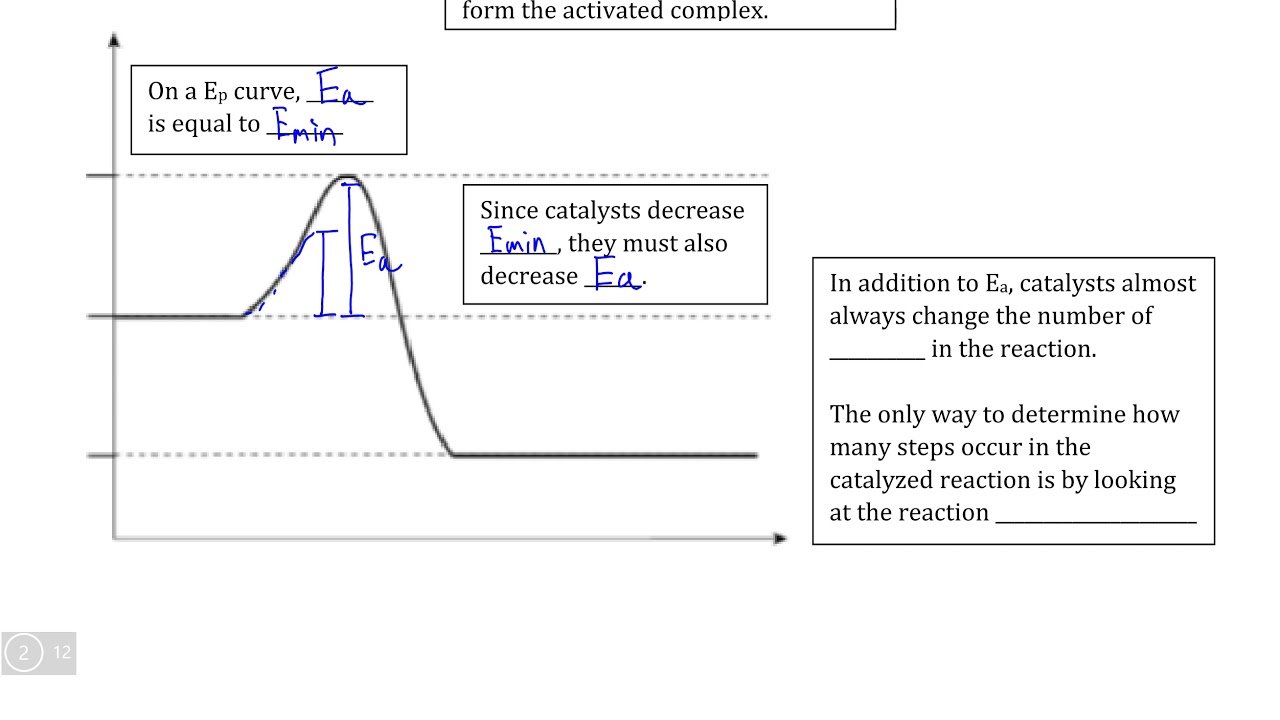
Progress Of A Reaction
When the colliding molecules possess the kinetic energy equal to Ea, the atomic configuration of species formed at this stage is different from the reactants as well as the products. This stage is called the activated state or transition state and specific configuration of this state is called activated complex or intermediate.
For example: In the reaction between H2 and I2 , activated complex has configuration in which H-H and I-I bonds are breaking and H-I bonds are forming.
This activated complex state is unstable transitions state of the reacting system which is mid-way between the reactants and the products. It has a very short life span and splits into the products to acquire stable state of lower energy.
Recommended Reading: What Does K Stand For In Math
Team Management And Stamina Effects
After an Ultimate Team game has started, the team management, e.g., position changes/formation changes, will not have an effect on chemistry. This means that you can freely modify your team after a match has started, and your players will not lose or gain any chemistry.
Stamina was also addressed towards the end of the article. EA mentions that stamina can have an effect on some statistics in the game, as it plays out. The statistics that are affected are the following:
- Acceleration