Solved Examples For You
Question: Why are covalent compounds not soluble in water?
Answer: Water molecules are not absolutely neutral. These molecules have a slight negative charge on the oxygen atom and slight positive charges on the hydrogen atoms. On the other hand, we know that the covalent compounds are made up of neutral molecules or molecules with slight charges. It is for this reason that these compounds are not attracted to water molecules strongly.
D: Nomenclature Of Anionsthe
Naming Anions of Sulfur, Nitrogen, and Phosphorus
- 1.For the -ide anions, how is the charge on the anion related to the electron configuration of the neutral element and the position of the element in the periodic table? How does the electron configuration of the element change when it forms the specified anion?
- 2.In the series of anions of the same element , what changes in the formula of the anion in going from the -ide anion to the -ite and the -ate anion?
- 3.What trend do you see in the number of oxygen atoms in the -ate forms of the anions to the -ite forms of the anions? What trend do you see in the charge of the -ate and the -ite anions?
- 4.Write a statement that describes how you could predict the charge on the anion of an element that would have the -ide ending .
- 5.The general name for the collection of -ate and -ite anions is oxyanion. Explain why these ions are called oxyanions.
- 6.Write a statement that would describe how to determine the formula of the -ite anion of an element from the formula of the -ate anion of that element.
Are Covalent Bonds Strong Or Weak
Covalent bonds are strong a lot of energy is needed to break them. Substances with covalent bonds often form molecules with low melting and boiling points, such as hydrogen and water.
Are covalent bonds hard?
Covalent bonds are extremely strong, so covalent solids are very hard. Generally, covalent solids are insoluble due to the difficulty of solvating very large molecules.
Why are covalent compounds not conductive to electricity?
Because there are no free electrons or ions in the water dissolved covalent compounds cant conduct electricity. Similarly, covalent compounds arent conductive in pure form either. Can metallic compounds conduct electricity?
Also Check: What Does And Mean In Math
The Different Kinds Of Compounds
Chemical compounds are generally grouped into one of two categories: covalent compounds and ionic compounds. Ionic compounds are made up of electrically charged atoms or molecules as a result of gaining or losing electrons. Ions of opposite charges form ionic compounds, usually as a result of a metal reacting with a nonmetal.
Covalent, or molecular, compounds generally result from two nonmetals reacting with each other. The elements form a compound by sharing electrons, resulting in an electrically neutral molecule.
Covalent Bonding In Carbon Atom
As per the electronic configuration of Carbon, it needs to gain or lose 4 electrons to become stable, which seems impossible as:
- Carbon cannot gain 4 electrons to become C4-, because it will be tough for 6 protons to hold 10 electrons and so the atom will become unstable.
- Carbon cannot lose 4 electrons to become C4+ because it would require a large amount of energy to remove out 4 electrons and also the C4+ would have only 2 electrons held by proton, which will again become unstable
Carbon cannot gain or donate electrons, so to complete its nearest noble gas configuration, it shares electron to form a covalent bond.
Also Check: What Is Experimental Research In Psychology
Hierarchical Structure Of The Atom
There are four hierarchical levels that describe the position and energy of the electrons an atom has. Here they are listed along with some of the possible values they can have:
Principal energy levels are made out of sublevels, which are in turn made out of orbitals, in which electrons are found.
Characteristics Of Covalent Compounds
Compounds that contain covalent bonds exhibit different physical properties than ionic compounds. Because the attraction between molecules, which are electrically neutral, is weaker than that between electrically charged ions, covalent compounds generally have much lower melting and boiling points than ionic compounds . For example, water boils at 100 °C while sodium chloride boils at 1413 °C. In fact, many covalent compounds are liquids or gases at room temperature, and, in their solid states, they are typically much softer than ionic solids. Furthermore, whereas ionic compounds are good conductors of electricity when dissolved in water, most covalent compounds, being electrically neutral, are poor conductors of electricity in any state. The attraction between molecules will be discussed in more detail in Section 8.1
Example \
Is each compound formed from ionic bonds, covalent bonds, or both?
-
The elements in \ are a metal and a nonmetal, which form ionic bonds.
- Answer b
-
Because sodium is a metal and we recognize the formula for the phosphate ion, we know that this compound is ionic. However, within the polyatomic phosphate ion, the atoms are held together by covalent bonds, so this compound contains both ionic and covalent bonds.
- Answer c
-
The elements in \ are both nonmetals, rather than a metal and a nonmetal. Therefore, the atoms form covalent bonds.
Exercise \
Is each compound are formed from ionic bonds, covalent bonds, or both?
Don’t Miss: What Is Iupac In Chemistry
Types Of Covalent Bond: Electronegativity
The second category of covalent bond is based on electronegativity.
Electronegativityis the tendency for elements to attract/gain electrons.
Elements with the largest electronegativity are near the top right of the periodic table while elements with the smallest electronegativity are near the bottom left , as shown below:
Fig.4-Table of electronegativities
The two types of covalent bonds in this category are:
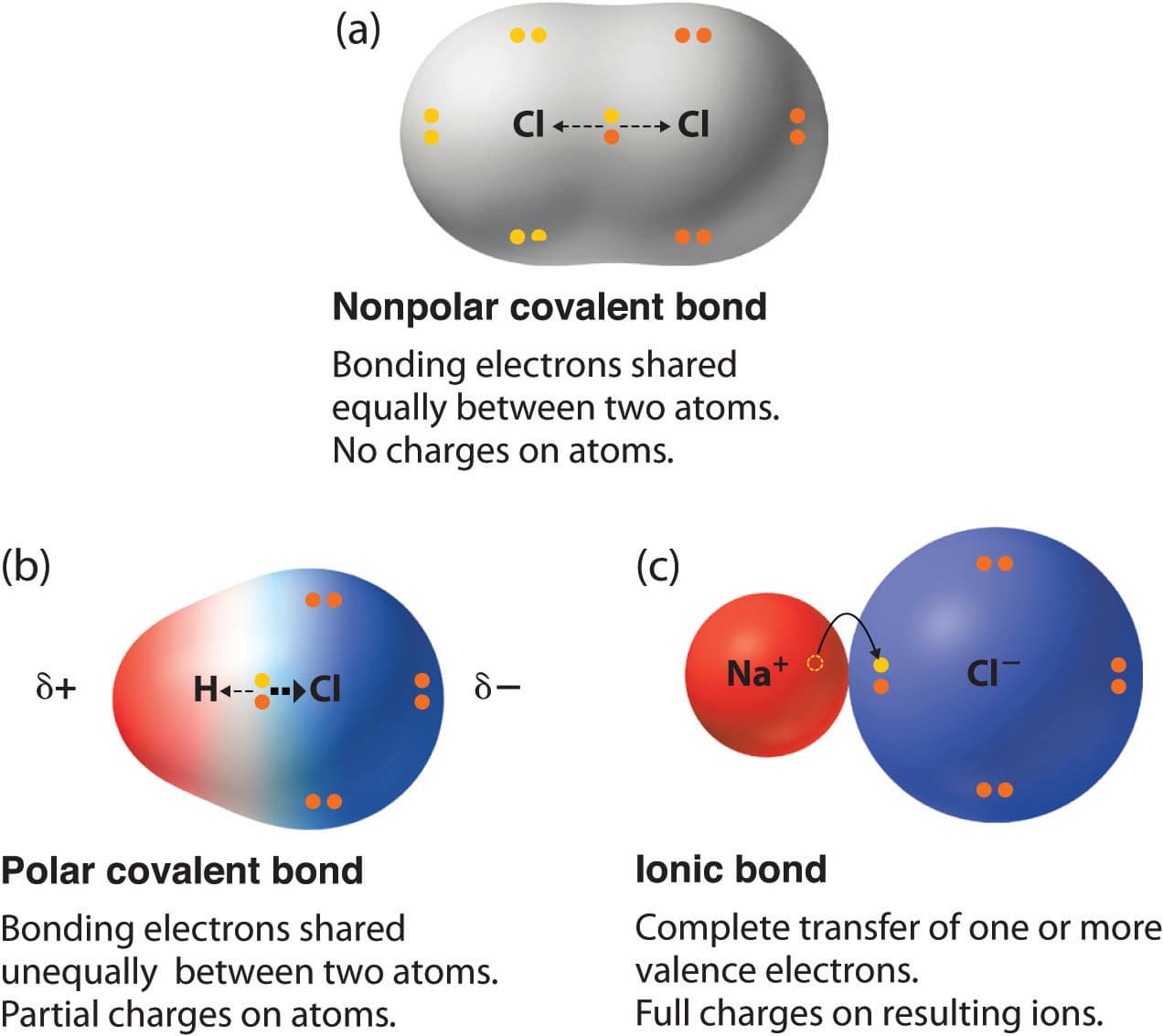
- Non-polar covalent
Here, “polarity” refers to the difference in electronegativity between elements. When one element has a significantly higher electronegativity , the bond is considered polar.
What happens is the electrons are attracted to this more electronegative element, which causes an uneven distribution of electrons. This in turn causes the side with more electrons to be slightly negatively charged , and the side with fewer electrons to be slightly positively charged
For example, below is HF , which is a polar covalent compound:
Fig.5-Hydrogen fluoride has a polar covalent bond
The separation of these charges is called a dipole.
In non-polar covalent bonds, there is a small enough difference in electronegativity , that is distribution of charge doesn’t occur, so there is no polarity. An example of this would be F2.
Lewis Formulation Of A Covalent Bond
The idea that two electrons can be shared between two atoms and serve as the link between them was first introduced in 1916 by the American chemist G.N. Lewis, who described the formation of such bonds as resulting from the tendencies of certain atoms to combine with one another in order for both to have the electronic structure of a corresponding noble-gas atom.
In Lewis terms a covalent bond is a shared electron pair. The bond between a hydrogen atom and a chlorine atom in hydrogen chloride is formulated as follows:
In a Lewis structure of a covalent compound, the shared electron pair between the hydrogen and chlorine ions is represented by a line. The electron pair is called a bonding pair the three other pairs of electrons on the chlorine atom are called lone pairs and play no direct role in holding the two atoms together.
Each atom in the hydrogen chloride molecule attains a closed-shell octet of electrons by sharing and hence achieves a maximum lowering of energy. In general, an incomplete shell means that some attracting power of a nucleus may be wasted, and adding electrons beyond a closed shell would entail the energetic disadvantage of beginning the next shell of the atom concerned. Lewiss octet rule is again applicable and is seen to represent the extreme means of achieving lower energy rather than being a goal in itself.
Next, one bonding pair is added between each linked pair of atoms:
Finally, each bonding pair is represented by a dash:
Recommended Reading: How To Study Ap Human Geography
Introduction To Covalent Molecules And Compounds
Just as an atom is the simplest unit that has the fundamental chemical properties of an element, a moleculeis the simplest unit that has the fundamental chemical properties of a covalent compound. Thus, the term molecular compound is used to describe elements that are covalently bonded and to distinguish the compounds from ionic compounds. Some pure elements exist as covalent molecules. Hydrogen, nitrogen, oxygen, and the halogens occur naturally as the diatomic molecules H2, N2, O2, F2, Cl2, Br2, and I2 in Figure 4.1). Similarly, a few pure elements exist as polyatomic molecules, such as elemental phosphorus and sulfur, which occur as P4 and S8 in Figure 4.1).
Figure 4.1 Elements That Exist as Covalent Molecules. Several elements naturally exist as diatomic molecules, in which two atoms are joined by one or more covalent bonds to form a molecule with the general formula E2. A few elements naturally exist as polyatomic molecules, which contain more than two atoms. For example, phosphorus exists as P4 tetrahedraregular polyhedra with four triangular sideswith a phosphorus atom at each vertex. Elemental sulfur consists of a puckered ring of eight sulfur atoms connected by single bonds. Selenium is not shown due to the complexity of its structure.
How to Recognize Covalent Bonds
Double And Triple Covalent Bonds
Covalent bonding occurs when electrons are shared between atoms. Double and triple covalent bonds occur when four or six electrons are shared between two atoms, and they are indicated in Lewis structures by drawing two or three lines connecting one atom to another. It is important to note that only atoms with the need to gain or lose at least two valence electrons through sharing can participate in multiple bonds.
Read Also: How Are Biology And Technology Related
Properties Of Molecular Compounds
Molecular compounds have many properties that differ from ionic compounds. Some of the generalizations for this group include much lower melting and boiling points when compared with their ionic counterpoints. For example, water has a melting point of 4oC and a boiling point of 100oC compared with NaCl that has a melting point of 801oC and a boiling point of 1,413oC. This is because the full charges created in ionic bonds have much stronger attractive force than the comparatively weak partial charges created in covalent molecules. thus, ionic compounds tend to form very strong crystalline lattice structures due to the repeating charges of the cation and anion components. Covalent compounds, on the otherhand, do not typically have such well-structured 3-dimensional shapes. Thus they tend to be more brittle and break more easily when in solid form, and many are found in liquid and gas phases. In addition, due to their lack of charges, they tend to be poor electrical and thermal conductors. Many are also insoluble in water due to their nonpolar nature .
Table 4.1 shows common differences between covalent and ionic compounds.
Table 4.1 Comparison of Ionic and Covalent Compounds
What Is A Covalent Compound
Covalent compounds are the ones having strong intra-molecular bonds. This is because the atoms within the covalent molecules are very tightly held together. Each molecule is indeed quite separate and the force of attraction between the individual molecules in a covalent compound tends to be weak.
We require very little energy in separating the molecules. This is because of the attractive forces between the molecules with the absence of overall electric charge. Covalent compounds are usually gaseous molecules at room temperature and pressure. They might also be liquids with low relatively low boiling points.
These characteristics could be attributed to their weak intermolecular forces which hold these atoms together. However, we also have a lot of solid covalent compounds. They have low melting points. However, it is interesting to note that a small number of these have a completely different structure. They form huge structures where a huge number of atoms are held together. This is possible due to the presence of shared electrons.
These giant molecular structures are basically lattices made up of molecules which are held together by covalent bonds structure. These covalent bonds are very strong. They also tend to be very hard with high melting points which are different from most of the covalent compounds. The example of this kind of covalent compounds includes diamond and graphite of carbon atom network. They also include silica of silicon and oxygen atoms network.
Recommended Reading: What Is Psychoanalysis In Psychology
How Does A Polar Covalent Bond Conduct Electricity
Out of these ,polar covalent compounds conduct electricity due to charge separation. Basically in polar covalent compounds the participating elements acquire a partial positive and negative charge due to huge electronegativity difference. That is responsible for them conducting electricity as they act as a good electrolyte.
How are ionic compounds used to conduct electricity?
Unlike in metals, the chemical bonding in liquids does not allow for electrons to move freely. This means we have to introduce charges into the water before it can start conducting. Certain compounds dissolve in water, they do so by dissociating or breaking up their bonds.
B: Predicting The Formula Of An Ionic Compound
- Why are parentheses needed in the formulas with multiple polyatomic ions in the compound?
- 6.Consider a cation with a 4+ charge and an anion with a 2 charge. How many cations and how many anions would be needed for an ionic compound formed between these two ions?
- 7.Write a rule that will allow you to predict the numbers of cations and anions present in the formula of an ionic compound. Make a list of what you need to know to be able to write the formula of an ionic compound.
You May Like: How Did Geography Affect Where Rome Was Located
Determining Covalent Bond Length
Bond length is the distance between the nuclei of elements in a bond
Covalent bond length is determined by bond order.
Bond order is the number of electron pairs shared between two bonded elements.
The higher the bond order, the shorter the bond. The reason why larger bonds are shorter is that the attractive forces between them are stronger.
When looking at diatomic compounds, the bond order is simply equal to the number of bonds . However, for compounds with more than two atoms, the bond order is equal to the total number of bonds minus the number of things bonded to that atom.
Let’s do a quick example to explain:
What is the bond order of carbonate ?
Fig.6–Structure of carbonate ion
Carbonate has a total of four bonds . However, carbon is only bonded to three things , so the bond order is 4/3.
Ionic And Covalent Bonding
There are primarily two forms of bonding that an atom can participate in: Covalent and Ionic. Covalent bonding involves the sharing of electrons between two or more atoms. Ionic bonds form when two or more ions come together and are held together by charge differences.
So how do you know what kind of bond an atom will make? That is actually the easy part. Metals and Non-Metals when combined make ionic compounds. Non-Metals when combined with other Non-Metals make covalent compounds. So all you need to be able to do is figure out what elements are Metals and which are Non-Metals. For that information we can use the periodic table:
Read Also: How To Solve Hard Physics Problems
C: Nomenclature For Covalent And Ionic Compounds
- 1.Is the first element written in the formula the more electronegative of the elements in the formula, or the less electronegative of the elements? Does this order change in the name of the compound? What does change in the name of the compound?
- 2.Describe how the number of elements in the formula is communicated in the name of the compound.
- 3.Consider the compounds in the table above with carbon and oxygen or with nitrogen and oxygen. Why is it important to communicate the numbers of each element in the name? Why would it not work, for example, to give the name of carbon oxide for a compound that consists of carbon and oxygen?
- 4.Is the cation or the anion written first in the formula? Does this order change in the name?
- 5.Is the number of cations or anions in the formula communicated in the name of the compound? Why do you think it is unnecessary to do this?
- 6.The names of the cations are the same as the names of the elements for the main group metals in the table, but not for the cations of copper and iron. What is the significance of the Roman numeral in the names of the cations of copper and iron?
Analyzing Nomenclature Rules
Naming Binary Covalent Compounds
There are many other naming schemes. Thereare naming schemes for acids, organic compounds and simple covalent compounds. You book covers simple covalent compounds in this chapter probablybecause it is so similar to the naming scheme for ionic compounds. Remember, ionic compounds are metal combined with a non-metal. A covalent compound is the combination of non-metals.
Rules for naming simple covalentcompounds:
1. Name the non-metal furthest to the left on the periodic tableby its elemental name.
2. Name the other non-metal by its elemental name and an -ideending.
3. Use the prefixes mono-, di-, tri-…. to indicate the numberof that element in the molecule.
4. If mono is the first prefix, it is understood and not written
N2O4 iscalled dinitrogen monoxide
CO2 is called carbondioxide
CO is called carbon monoxide
N2O is called dinitrogenmonoxide.
CCl4 is called carbontetrachloride
Here is a chart of those prefixes:
|
1 – mono |
Don’t Miss: What Is The Peter Principle In Psychology