Durban Girls College Durban South Africa
Chlorine: I decided to make the whole tile green-yellow as this is the colour of chlorine. The gas is seeping up from the symbol and atomic number of the element as chlorine is a gas at room temperature. The tap and glass of water signify how helpful chlorine is for water purification, while the salt shaker shows that chlorine is also found in the form of a salt with sodium. The last and biggest image, a gas mask, symbolizes how chlorine gas was used as a deadly gas in World War I, which stopped soldiers from breathing and damaged their eyes and lungs.
Original artwork by Rebecca Plumble, grade 8 student teacher: Helen McCready, Durban Girls College, Durban, Kwazulu-Natal, South Africa
Education Apprenticeship And Poetry
After Davy’s father died in 1794, Tonkin apprenticed him to John Bingham Borlase, a surgeon with a practice in Penzance. While becoming a chemist in the ‘s dispensary, he began conducting his earliest experiments at home, much to the annoyance of his friends and family. His older sister, for instance, complained his corrosive substances were destroying her dresses, and at least one friend thought it likely the “incorrigible” Davy would eventually “blow us all into the air.” In 1797, after he learned French from a refuge priest, Davy read ‘s Traité élémentaire de chimie. This exposure influenced much of his future work, which can be seen as reaction against Lavoisier’s work and the dominance of French chemists.
As a poet, over one hundred and sixty manuscript poems were written by Davy, the majority of which are found in his personal notebooks. Most of his written poems were not published, and he chose instead to share a few of them with his friends. Eight of his known poems were published. His poems reflected his views on both his career and also his perception of certain aspects of human life. He wrote on human endeavours and aspects of life like death, metaphysics, geology, natural theology and chemistry.
How Does Chlorine React With Alkalis
The reaction between chlorine and an alkali such as sodium hydroxide doesnt give us chloric acid, but instead sodium chlorate, which we met above. Again, this is used to treat wastewater, but it is also the active ingredient in household bleach.
Solutions containing sodium chlorate are particularly useful for removing stains on cutlery caused by tannins in tea.
The equation is given below:
This is another example of a disproportionation reaction. Chlorine atoms are both oxidised to make sodium chlorate and reduced to make sodium chloride.
The reaction between chlorine and sodium hydroxide. Anna Brewer, StudySmarter Originals
You might have heard of Ignaz Semmelweis, one of the founders of modern hygiene practices in hospitals and surgeries. Semmelweis noticed that a strangely high proportion of babies delivered by certain doctors were dying soon after birth, compared to those delivered by midwives. These doctors often came straight from dissecting rooms where they were working on cadavers. Semmelweis proposed that they were carrying ‘cadaveric particles’ that transmitted decay from dead corpse to newborn child. He found that a simple solution of chlorine dissolved in water was an effective way of stopping the spread of disease.
Recommended Reading: Geometry Segment And Angle Addition Worksheet
Folk And Pass Medicine
was used as a beginning in the early 1900s. However, it fell into disfavour in the 1940s due to the rising popularity of safer and more efficient sedatives and when some heart patients died after using a salt substitute . Like and , it was used as a treatment for .
Bromide compounds, especially , were frequently used as sedatives in the 19th and early 20th centuries. Their use in over-the-counter sedatives and headache remedies in the United States extended to 1975 when bromides were withdrawn as ingredients due to . This use gave the word “bromide” its colloquial connotation of a comforting .
It has been said that during , British soldiers were given bromide to curb their sexual urges. mentions a soldier being given bromide as a sedative for nervous exhaustion and overwork in his play Fame and the Poet .
Bromide salts are used in as mild agents to generate in situ
The bromide ion is and as bromide salt, is used in veterinary medicine in the US. The kidneys excrete bromide ions. The half-life of bromide in the human body is long compared with many pharmaceuticals, making dosing challenging to adjust . Bromide ion concentrations in the are about 30% of those in blood and are strongly influenced by the body’s chloride intake and metabolism.
Are There Other Uses For Chlorine
The main purpose of chlorination is to disinfect water, but it also has many other benefits. Unlike some of the other disinfection methods like ozonation and ultraviolet radiation, chlorination is able to provide a residual to reduce the chance of pathogen regrowth in water storage tanks or within the water distribution system. At times, distribution systems can be a fair distance from the storage tanks and in dead end sections or where water is not used pathogens may re-grow if a proper residual is cannot be maintained in the treated water sent out for consumption. This results in poor water quality as well as slime and biofilms in the distribution systems that will end up contaminating the clean, treated water being distributed. Many government environmental bodies have set guidelines or standards for the amount of chlorine residual that must be present at all points in the system. The guidelines for each province are shown in the table below.
In addition to providing a residual, adding chlorine to water will also: oxidize iron, manganese, taste and odour compounds, remove colour in the water, destroy hydrogen sulphide, and aid other water treatment processes, such as sedimentation and filtration. Oxidizing soluble reduced iron and manganese will result in particle formation as oxidized iron and manganese are not soluble in water.
Read Also: Michael Jackson’s Kids Biological
Combines Easily To Form These Very Well
- Sodium chloride Known widely as common table salt, sodium chloride is an important component of the diets of both people and animals. Sodium chloride is the primary feedstock of chlorine for the chemical industry.
- Hydrochloric acid A strong acid, hydrochloric acid is extremely useful for titration, reacting with unknown bases to determine their composition. Hydrochloric acid also has many uses including processing steel and food products like gelatin and sugar, and producing batteries. In humans, it is produced in our stomachs to help digest food.
- Polyvinyl chloride Most PVC compounds are made using sodium chloride. They are extremely useful thermoplastics that can replace rubber or metal pipes. Additionally, they are very lightweight and are also used for many purposes in the healthcare industry, such as tubing.
- Magnesium chloride Found in seawater and serves as a natural source of metal magnesium. Magnesium is not only used to create alloys for manufacturing processes, but it is also the fourth most prevalent element in the human body and essential for nutrition.
Discover all the products made possible by chlorine chemistry through our chlorine and sodium hydroxide product trees.
How Can People Be Exposed To Chlorine
Given the ubiquity and volume of chlorine in industrial and commercial locations, widespread exposures could occur from an accidental spill or release, or from a deliberate terrorist attack.
Because chlorine is a gas at room temperature, exposure occurs via inhalation. People may also be exposed to chlorine through skin or eye contact, or through ingestion of chlorine-contaminated food or water.
Read Also: Ccl4 Molecular Structure
What Is Chlorines Mechanism Of Action
The health effects of chlorine are primarily due to its corrosive properties. The strong oxidizing effects of chlorine cause hydrogen to split from water in moist tissue, resulting in the release of nascent oxygen and hydrogen chloride which produce corrosive tissue damage. The oxidation of chlorine may also form hypochlorous acid, which will penetrate cells and react with cytoplasmic proteins to destroy cell structure.
Is Chlorine A Poisonous Gas
Gaseous chlorine is poisonous and is listed as an irritant to the lungs. It has intermediate water solubility with the ability to cause upper and lower respiratory tract acute damage. Due to its strong smell, chlorine gas can be easily detected.
Learn more about the atomic weight, electron configuration and the uses of Cl from the expert faculties at BYJUS.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
Don’t Miss: What Is The Difference Between Electron Geometry And Molecular Geometry
Producing Chlorine From Potassium Chloride
How is chlorine made from other compounds? Sometimes, the salt used is not sodium chloride, but rather potassium chloride . When KCl is used, the resulting products are chlorine, potassium hydroxide , and hydrogen gas.
Citation: Clark, J. . The Manufacture of Chlorine. Retrieved from Chemistry LibreTexts
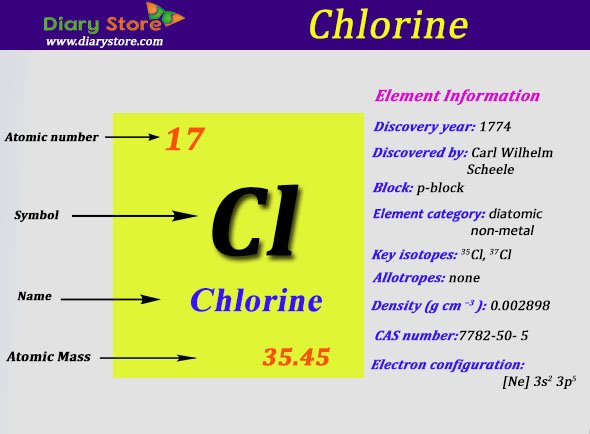
Fast Facts: The Element Chlorine
- Element Name: Chlorine
Electron Configuration: 3s2 3p5
Word Origin: Greek: khloros: greenish-yellow
Properties: Chlorine has a melting point of -100.98°C, boiling point of -34.6°C, density of 3.214 g/l, specific gravity of 1.56 , with a valence of 1, 3, 5, or 7. Chlorine is a member of the halogen group of elements and directly combines with almost all of the other elements. Chlorine gas is a greenish yellow. Chlorine figures prominently in many organic chemistry reactions, particularly in substitutions with hydrogen. The gas acts as an irritant for respiratory and other mucous membranes. The liquid form will burn the skin. Humans can smell as low an amount as 3.5 ppm. A few breaths at a concentration of 1000 ppm is usually fatal.
Uses: Chlorine is used in many everyday products. It is used for disinfecting drinking water. Chlorine is used in the production of textiles, paper products, dyes, petroleum products, medicines, insecticides, disinfectants, foods, solvents, plastics, paints, and many other products. The element is used to manufacture chlorates, carbon tetrachloride, chloroform, and in the extraction of bromine. Chlorine has been used as a chemical warfare agent.
In nature, chlorine is only found in the combined state, most commonly with sodium as NaCl and in carnallite and sylvite . The element is obtained from chlorides by electrolysis or via the action of oxidizing agents.
Element Classification: Halogen
Fusion Heat : 6.41
Evaporation Heat : 20.41
Read Also: Ccl4 Geometry
Are There Health Concerns With Chlorinating Water
Chlorine can be toxic not only for microorganisms, but for humans as well. To humans,chlorine is an irritant to the eyes, nasal passages and respiratory system. Chlorine gas must be carefully handled because it may cause acute health effects and can be fatal at concentrations as low as 1000 ppm. However, chlorine gas is also the least expensive form of chlorine for water treatment, which makes it an attractive choice regardless of the health threat.
In drinking water, the concentration of chlorine is usually very low and is thus not a concern in acute exposure. More of a concern is the long term risk of cancer due to chronic exposure to chlorinated water. This is mainly due to the trihalomethanes and other disinfection by-products, which are by-products of chlorination. Trihalomethanes are carcinogens, and have been the topic of concern in chlorinated drinking water. Chlorinated water has been associated with increased risk of bladder, colon and rectal cancer. In the case of bladder cancer, the risk may be doubled. Although there are concerns about carcinogens in drinking water, Health Canada’s Laboratory Centre for Disease Control says that the benefits of chlorinated water in controlling infectious diseases outweigh the risks associated with chlorination and would not be enough to justify its discontinuation. In Europe, however, chorination has been discontinued in many communities.
Pool Chlorine: What It Is How It Works & How To Use It

- |March 11, 2019
Lets face it, pool maintenance is no walk in the park, especially when it comes to learning the processes and ingredients that contribute to a clean and friendly pool.
If youre reading this, you probably know that chlorine is one of the most important ingredients so understanding what it is, how it works, why you need it, and how to use it effectively is a high priority.
This guide will arm you with all the pool chlorine knowledge you need to keep your pool water in pristine condition, so lets get right into it.
Skip to:
You May Like: Does Elton John Have Children
What Happens During The Test
You will usually have a blood sample taken at your doctorâs office or a lab. Chloride levels also can be checked with a urine test.
A lab tech will insert a needle into a vein in your arm to get the sample. Your arm may be a little sore where the blood was drawn. Some people become lightheaded for a few moments.
How Is Chlorine Used
Chlorine has a variety of uses. It is used to disinfect water and is part of the sanitation process for sewage and industrial waste. During the production of paper and cloth, chlorine is used as a bleaching agent. It is also used in cleaning products, including household bleach which is chlorine dissolved in water. Chlorine is used in the preparation of chlorides, chlorinated solvents, pesticides, polymers, synthetic rubbers, and refrigerants.
Read Also: Hrw Geometry Answers
Chlorine Chemical & Physical Properties
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
Chlorine is a chemical element with atomic number 17 and element symbol Cl. It is a member of the halogen group of elements, appearing between fluorine and bromine moving down the periodic table. At ordinary temperature and pressure, chlorine is a pale. greenish-yellow gas. Like other halogens, it is an extremely reactive element and strong oxidizer.
Reaction Of Chlorine With Oxygen
Chlorine doesnt normally react with oxygen. However, in the presence of UV light, chlorine can react with oxygen or ozone molecules to form the chlorine monoxide free radical, :
Like all radicals, this species is extremely reactive and can break down ozone in the ozone layer.
To find out more about how chlorine destroys ozone, check out Ozone Depletion.
You May Like: Geometry Dash Passwords
Chemistry As A Physical Science
Chemistry is typically considered a physical science, as defined by the Encyclopedia Britannica, because the study of chemistry does not involve living things. Most of the chemistry involved in research and development, such as making new products and materials for customers, falls within this purview.
But the distinction as a physical science becomes a bit blurry in the case of biochemistry, which explores the chemistry of living things, according to the Biochemical Society. The chemicals and chemical processes studied by biochemists are not technically considered “living,” but understanding them is important to understanding how life works.
Properties Of Sodium Chloride
Physical Properties of NaCl
Sodium chloride, a white crystalline solid, contains a density of 2.165 g/mL, a melting point of 801 °C, and a boiling point is about 1,413 °C. It is also available as aqueous solutions with different concentrations, which are known as saline solutions.
Chemical Properties of NaCl
Sodium chloride is a readily soluble compound in water and other polar solvents and is a stable solid. It decomposes only at high temperatures to produce toxic fumes of disodium oxide and hydrochloric acid .
Read Also: Holt Algebra 2 Chapter 7 Test Form A Answers
What Is Chlorine
Chlorine is the second lightest halogen and is represented as Cl. The atomic number of this chemical element is 17.
It appears as a pale yellow-green gas. Liquid chlorine can cause skin burn and chlorine in its gaseous form irritates the mucous membrane. Its position as per the periodic table is between fluorine and bromine. Its electronic configuration is 3s23p5. There are two isotopes of chlorine that are stable.
They are 37Cl and 35Cl. 36Cl is the stable radioisotope of chlorine. Sodium chloride is the most common compound of chlorine whereas the simplest is hydrogen chloride. Sodium chloride has a molecular formula NaCl whereas hydrogen chloride has a molecular formula HCl. It is highly reactive. Carl Wilhelm Scheele who was a Swedish chemist discovered Chlorine in the year 1774.
Why Is It O

I’m currently preparing my exam and while I did never think about this question during the course, it begins to confuse me right now…
Why is it the structure of $\ce -}$ like $\ce $ and not like $\ce $?
I know how to place the covalent bonds and the electrons, but I don’t know how to figure out the basic structure of the molecule. Is there a relatively simple rule to solve such relatively simple molecules?
Is there a relatively simple rule to solve such relatively simple molecules?
The most electropositive atom is usually at the center of a molecule because
- a “central atom” implies that there are multiple bonds to the central atom. If the central atom is relatively electropositive, then it will be better able to share its electrons and form bonds with other atoms, at least more so than an electronegative central atom would.
- electronegative atoms tend to carry multiple lone pairs of electrons. If this electronegative atom and all of its lone pairs were at the center of the molecular structure, then we would have many more destabilizing electron-electron repulsions, then if all of these lone pairs were on the periphery of the molecule.
Don’t Miss: My.hrw Algebra 1
Structure And Characteristics Of Inorganic Iodides
Iodide is one of the largest monatomic . It is assigned a radius of around 206 . For comparison, the lighter halides are considerably smaller: , , and fluoride . In part because of its size, iodide forms relatively weak bonds with most elements.
Most iodide salts are soluble in water, but often less so than the related chlorides and bromides. Iodide, being large, is less hydrophilic compared to the smaller anions. One consequence of this is that sodium iodide is highly soluble in acetone, whereas sodium chloride is not. The low solubility of and reflects the covalent character of these metal iodides. A test for the presence of iodide ions is the formation of yellow precipitates of these compounds upon treatment of a solution of or .
Aqueous solutions of iodide salts dissolve iodine better than pure water. This effect is due to the formation of the ion, which is brown:
- I + I2 I3