What Is Intermediate Free Radical
Free radicals are reaction intermediates formed due to the homolytic cleavage of a covalent bond containing carbon such that carbon gets an unpaired electron. In this process each atom takes away one of the two electrons forming a single covalent bond. It will produce two new species having an unpaired electron.
How Do You Identify Transition States
Transition state structures can be determined by searching for first-order saddle points on the potential energy surface of the chemical species of interest. A first-order saddle point is a critical point of index one, that is, a position on the PES corresponding to a minimum in all directions except one.
What Is The Difference Between Catalyst And Intermediate
June 15, 2022 Posted by Madhu
The key difference between catalyst and intermediate is that a catalyst is useful at the beginning of the reaction and is regenerated at the end, whereas an intermediate is formed during the chemical reaction and does not exist at the end of the reaction.
The terms catalyst and intermediate are very important in chemical reactions. A catalyst is a chemical compound that can increase the rate of a reaction without itself being consumed, whereas an intermediate is a molecule that forms from two or more reactants and undergoes further reaction to give final products.
Read Also: What Does Kw Equal In Chemistry
What Is Intermediate In Rate Of Reaction
A reaction intermediate is a chemical species that is formed in one elementary step and consumed in a subsequent step. The slowest step in a reaction mechanism is known as the rate-determining step. The rate-determining step limits the overall rate and therefore determines the rate law for the overall reaction.
Reaction Intermediates: Definition Examples & More
Reaction Intermediates: Reaction intermediates in organic chemistry refers to the high-energy, highly reactive, as well as short-lived molecule in a chemical reaction. It usually gets generated during a chemical reaction which gets stabilised to a stable molecule. All the types of reaction intermediates are having some common features. They are having low concentration with correspondence to reaction substrate and the final reaction product. Along with that, there are pieces of evidence of the chemical deposition of a chemical compound.
This article will help candidates find all the basis of organic reactive intermediates which will serve them a lot in their organic chemistry preparation for both the board and the competitive exams. Other than that, this article will also help candidates know the types of reactive organic intermediates and examples. Keep reading to know more!
You May Like: Big Ideas Math Geometry Chapter 2 Test
Definition Of An Intermediate
Intermediate is a comparatively long-lived species that can be experimentally detected and characterized. This means that an intermediate is an actual molecule or an ion that you can work with, sometimes even isolate. Thats you freezing in space as youve landed on one foot while hopping in a park. Its not the beginning of your journey , nor it is the end . Instead, its a relatively stable midpoint .
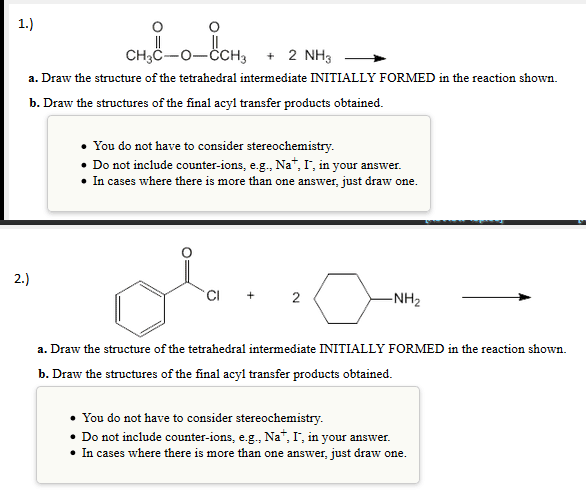
It is also very important to remember that a reaction doesnt have to have an intermediate! You may or may not have one depending on the nature and the mechanism of the reaction. There are plenty of single-step reactions that have no intermediates at all. A typical first semester organic chemistry example is an SN2 reaction that involves no intermediate whatsoever .
You will always have a transition state though! Every reaction, no matter how simple it might be, has a transition state. For instance, lets consider the following bromine dissociation reaction giving us two bromine radicals:
The two bromine atoms didnt just magically split and appeared in different places in space. They first had to stretch the bond to the brink of breaking and then break apart:
So, the transition state in this reaction is an elongated bond which is almost, but not quite, broken.
In a nutshell, youll have a transition state for every single step in your reaction. So, you can always check the number of transition states by counting the steps in the mechanism and vice-versa.
Main Applications Of Pharmaceutical Intermediates
The research on pharmaceutical intermediates is mainly reflected in the synthesis of heterocyclic compounds, fluorine-containing compounds, chiral compounds, biological compounds, etc.
Heterocyclic compounds, which are widely used in medicines, pesticides and veterinary drugs and intermediates, are mostly the parent . At present, losartan potassium tablets, amlodipine, etc., which are widely used in clinical treatment of hypertension, are all heterocyclic compounds.
Fluorine-containing compounds, introducing new elements into pharmaceutical intermediates for modification, the most successful should be fluorine. Organic fluorine-containing compounds have broad application prospects in medicine. At present, the best-selling anti-infective drug on the market, fluoxetine, is a fluorine-containing organic compound. In addition, fluorine anesthetics such as halothane, penthrane, ethrane, isoflurane, etc. are all anesthesia. Highly potent, non-post-anaesthetic organofluoride. 5-Fluorouracil, fluoxymesterone and others have been used to treat cancer, haloperidol for sedatives, sulindac for rheumatoid arthritis, and hydroflumethizaide Used as a diuretic, etc.
Pharmaceutical intermediate engineering technology is an important means to change the level of pharmaceutical intermediates, and it is also the key to the development of pharmaceutical intermediates, so it is imperative to carry out research on it.
âINFORMATION
Recommended Reading: How To Support Ell Students In Math
What Is An Intermediate In Organic Chemistry
Reaction Intermediates: Meaning, Types, Examples. Reaction Intermediates in Organic Chemistry: Know how many types of reaction intermediates are there in this article above.
Nitrenes are neutral monovalent nitrogen species that have two unshared pairs of electrons and are linked to only one monovalent atom or group. Like carbenes, nitrenes exist in both singlet and triplet states. The triplet state is the more stable of the two. Because nitrene is an electron-deficient species, it is highly reactive.
Video advice: Reaction Intermediates-I
Description of reaction intermediates such as free radicals, carbonation and carbanions by Dr Preet Jaggi
A reaction intermediate, also known as an intermediate, is a molecular entity generated from reactants that reacts further to produce the immediately seen products of a chemical process. Except for the penultimate stage, which produces the final product, each of these phases produces an intermediate. Reactive intermediates are rarely isolated and usually have a brief life span. They also do not remain in the product combination due to their limited lifespan.
Determining The Geometry Of A Transition State
Transition state structures can be determined by searching for first-order saddle points on the potential energy surface of the chemical species of interest. A first-order saddle point is a critical point of index one, that is, a position on the PES corresponding to a minimum in all directions except one. This is further described in the article geometry optimization.
Don’t Miss: What Is Buffer Solution In Chemistry
What Is An Intermediate In A Reaction Example
The term intermediate means something different in the chemical industry, referring to a stable product of a chemical reaction that is then used as a starting material for another reaction. For example, benzene and propylene may be used to make the intermediate cumene. Cumene is then used to make phenol and acetone.
Definition Of A Transition State
Transition state is the highest point on the reaction coordinate diagram. Those are the peaks or the hills in the picture. A more strict definition is that a transition state is a molecular entity that has a lifetime no longer than a vibration that exhibits some structural characteristics of both the reactants and the products. As I couldnt find the official definition, so Ive adapted this one from the Anslyn & Doughertys Modern Physical Organic Chemistry book. I think, it describes the transition state the best:
- its neither the reactant nor it is a product
- it resembles both to some extent, and
- its not something that can be isolated
The best analogy I can think to describe a transition state is this: imagine yourself merrily hopping down the alley in a park. That moment in time, when youre up in the air in the mid-jump is your transition state! You cannot catch that state when youre suspended in the midair, its neither your right leg, nor it is your left leg step its something in between. Same applies to transition states: they are somewhere in between.
Don’t Miss: Discovering Geometry Chapter 3 Answers
Is Intermediate Higher Than Advanced
â If you arenât experienced or knowledgeable but you are fast, you are an Intermediate. â If you arenât fast, but youâve been in the sport for a while and are knowledgeable about the sport, you are an Intermediate. However, if you are both experienced AND fast, itâs time to face facts: You are Advanced.
Summary Catalyst Vs Intermediate
The key difference between catalyst and intermediate is that a catalyst is added at the beginning of the reaction and regenerated at the end of the reaction whereas an intermediate is formed during the reaction and is not regenerated at the end of the reaction.
Reference:
1. Helmenstine, Anne Marie, Ph.D. What Is a Reaction Intermediate? ThoughtCo, Aug. 27, 2020.
Image Courtesy:
2. Hess Cycle Diagram By SGDWN Own work via Commons Wikimedia
Don’t Miss: What Is O2 In Chemistry
What Makes An Intermediate Stable
In chemistry, a reactive intermediate or an intermediate is a short-lived, high-energy, highly reactive molecule. When generated in a chemical reaction, it will quickly convert into a more stable molecule. Only in exceptional cases can these compounds be isolated and stored, e.g. low temperatures, matrix isolation.
What To Expect On The Exam
There are 3 common types of a question youre going to see on the test that deal with transition states.
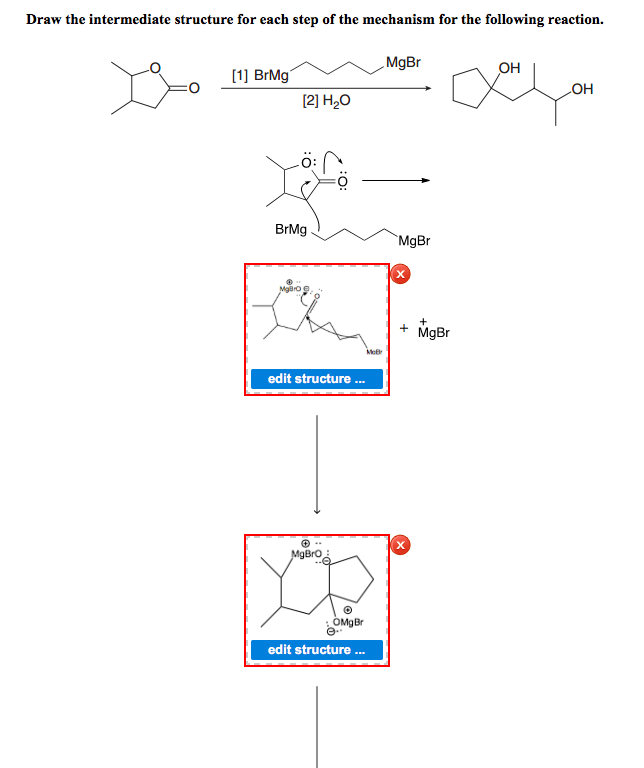
Type 1 is a question that shows you a reaction diagram and asks for the transition states and intermediates. For instance:
As you remember, the intermediate is the valley in the diagram, so it must be the option C in this case.
Type 2 is to count the number of intermediates, transitions states, or the mechanistic steps in the reaction based on the diagram. The reaction diagram above has 2 intermediates and 3 transition states, so it is a 3-step reaction.
Finally, the last question you can expect is a question about the shape or a nature of the transition state itself. We know that the transition state is something in-between the reagents and products/intermediate. To estimate the exact nature of the transition state, though, well need to use a principle known as Hammonds postulate, which is a whole topic on its own, and I will talk about some other time.
Recommended Reading: Does Adderall Permanently Change Brain Chemistry
How Do You Identify An Intermediate
1:365:14How To Identify The Intermediate & Catalyst In a Reaction MechanismYouTubeStart of suggested clipEnd of suggested clipSo d is the intermediate it’s produced first and then it’s consumed later the intermedia doesn’tMoreSo d is the intermediate it’s produced first and then it’s consumed later the intermedia doesn’t show up at the beginning or at the end of the reaction. It’s somewhere in the middle of the reaction.
What Is A Catalyst
A catalyst is a chemical compound that can increase the rate of a reaction without itself being consumed. Therefore, this compound can continue to act repeatedly. Due to this reason, only a small amount of catalyst is required for a certain chemical reaction.
A catalyst provides an alternative pathway for a chemical reaction by reducing the activation energy of a reaction. Here, the catalyst combines with the reactant to create an intermediate product, and after the completion of the required reaction, the catalyst leaves the intermediate and regenerates.
There are two types of catalysts they are homogeneous and heterogeneous catalysts. In homogeneous catalysts, the molecules are in the same phase as reactant molecules. However, in heterogeneous catalysts, the molecules are in a different phase to that of reactant molecules. Enzymes are a good example of biological catalysts.
You May Like: What Does It Mean To Have Chemistry With Someone
What Are Intermediates And What Do They Do
Posted on February 15, 2019 by MDMadmin – News
An intermediate is a molecule that is formed from two or more reactants and then reacts further to give products. Most chemical reactions require more than one step, and an intermediate is the product of each step, except for the last one, after which the final products are produced. Intermediates very rarely remain in the product mixture due to the short time that they exist. They are seldom isolated, and so usually end up reacting with other chemicals in the reaction to eventually produce the final products. An example of a chemical reaction would be A+B = C+D. In reality, the reaction is more likely to be something like this A+B = X*, X* = C+D, in which X* is the intermediate. There can be a high number of intermediates in every reaction, and theyre sometimes difficult to identify due to how short-lived they are.
An example of an intermediate in the chemical industry is cumene. The term intermediate in the chemical industry usually means a product of a reaction that is only beneficial when used as a precursor chemical for another industry. Cumene is made from benzene and propylene, and is then used to produce acetone. Cumene, without additional reactions, has very little value and no real use, which makes it an intermediate instead of a useful chemical product.
Contact
The Prospect Of Pharmaceutical Intermediates
Medicine is a special commodity with all the characteristics of fine chemicals. The development of medicine is inseparable from intermediates, which are the basis for the development of medicine. Pharmaceutical intermediates are the general term for all the compounds in the synthesis and manufacture of pharmaceuticals, pesticides, and veterinary drugs before they become drugs. Fine chemicals, including organic pharmaceutical intermediates, are an important part of the chemical industry, and their level of development is a sign of a country’s chemical modernization level. Over the years, the fine chemical industry has been the focus of investment and fierce competition from countries all over the world. The global pharmaceutical industry has thus made unprecedented progress, and the world pharmaceutical market continues to exhibit high growth characteristics. Due to the characteristics of high investment and high benefit in the research and development of new drugs, in order to maintain their high growth rate, major pharmaceutical companies increasingly focus on the research and development of new drugs, new drug products and their market development. Therefore, the development of pharmaceutical intermediates has broad market prospects.
Recommended Reading: Algebra 2 Chapter 3 Test Answer Key
Do Intermediates Increase The Rate Of Reaction
A stabilized intermediate means lower activation energy and as mentioned above, lower activation energy means lower activation barrier so the reactants can form products at a faster rate. The result is generally a very large increase in reaction rates on the order of millions of times.
Research On Chemical Intermediates
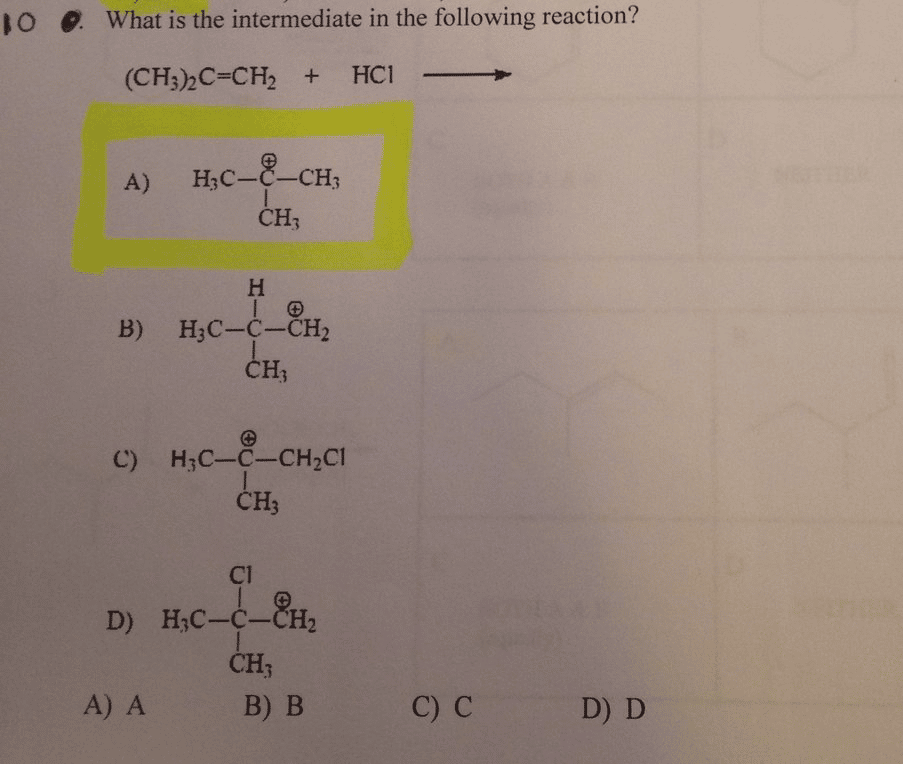
Research on Chemical Intermediates publishes current research articles and concise dynamic reviews on the properties, structures and reactivities of intermediate species in all the various domains of chemistry.
The journal also contains articles in related disciplines such as spectroscopy, molecular biology and biochemistry, atmospheric and environmental sciences, catalysis, photochemistry and photophysics. In addition, special issues dedicated to specific topics in the field are regularly published.
- Publishes current research articles and concise dynamic reviews
- Reports on the properties, structures and reactivities of intermediate species in all the various domains of chemistry
- Features articles in related disciplines and special issues dedicated to specific topics in the field
- 97% of authors who answered a survey reported that they would definitely publish or probably publish in the journal again
Don’t Miss: Why Is Purity Important In Chemistry
From One Component: Cyclization Of Reactive Intermediates
Reactive intermediates such as nitrenes, carbenes, and radicals have been used in cyclization reactions which produce aziridines and azirines. Intermolecular aziridination reactions with nitrenes were covered in Section 1.01.9.2. Vinylnitrenes may undergo cyclization to 2H-azirines , as shown in Scheme 78. This reaction has been covered in several reviews B-82MI 101-01, B-83MI 101-02, 84CHEC-I47, B-84MI 101-03, 87CHE1037. Vinylnitrenes may be generated by the thermolysis or photolysis of vinyl azides , or by the base-promoted loss of HX from oxime O-sulfonates or hydrazonium salts Me3 I). The latter process is known as the Neber reaction . While there has been considerable debate about the intermediacy of vinylnitrenes in both processes, these reactions are conveniently discussed in this Section.
Scheme 78.
Scheme 79.
P.G. Wells, L.M. Winn, in, 2010
What Is Reaction Intermediate Explain With Suitable Example
In the chemical industry, the term intermediate may also refer to the product of a reaction that is itself valuable only as a precursor chemical for other industries. A common example is cumene which is made from benzene and propylene and used to make acetone and phenol in the cumene process.
Don’t Miss: Geometry Sin Cos Tan Chart
What Is The Meaning Of Intermediate In Chemistry
An intermediate is a molecule that is formed from two or more reactants and then reacts further to give products. Most chemical reactions require more than one step, and an intermediate is the product of each step, except for the last one, after which the final products are produced.
What Are Intermediates In A Reaction Mechanism
A reaction intermediate is a chemical species that is formed in one elementary step and consumed in a subsequent step. The slowest step in a reaction mechanism is known as the rate-determining step. The rate-determining step limits the overall rate and therefore determines the rate law for the overall reaction.
Read Also: What Is The Definition Of Absolute Value In Math Terms
Which Reaction Is Intermediate In Rate
A reaction intermediate is a chemical species that is formed in one elementary step and consumed in a subsequent step. The slowest step in a reaction mechanism is known as the rate-determining step. The rate-determining step limits the overall rate and therefore determines the rate law for the overall reaction.