Protons In The Cytosol
- Protons or H+ are present in the cytoplasm of a cell that helps to maintain the pH of the solution.
- These protons are attracted by the oxygen atom of water molecules and thus play an essential role in the transmembrane transport of molecules.
- The membranes are permeable to water but not to the protons. As a result water molecules can freely across the membrane.
- Due to the attraction between the water molecules and protons, a proton motive force is created.
- The proton motive force can then be used for the transport of a variety of substances across the membrane.
Preparation From Constituent Ingredients
It is common practice in laboratories to make a solution directly from its constituent ingredients. There are three cases in practical calculation:
- Case 1: amount of solvent volume is given.
- Case 2: amount of solute mass is given.
- Case 3: amount of final solution volume is given.
In the following equations, A is solvent, B is solute, and C is concentration. Solute volume contribution is considered through the ideal solution model.
- Case 1: amount of solvent volume VA is given. Solute mass mB = C VA dA /
- Case 2: amount of solute mass mB is given. Solvent volume VA = mB
- Case 3: amount of final solution volume Vt is given. Solute mass mB = C Vt /100 Solvent volume VA= mB
- Case 2: solute mass is known, VA = mB 100/C
- Case 3: total solution volume is known, same equation as case 1. VA=Vt mB = C VA /100
Example: Make 2 g/100mL of NaCl solution with 1 L water Water . The density of the resulting solution is considered to be equal to that of water, statement holding especially for dilute solutions, so the density information is not required.
- mB = C VA = g/mL Ã 1000 mL = 20 g
Nutrients In The Soil
Plants need access to nutrients and minerals in the soil to survive. To get these nutrients and minerals, plants must diffuse the nutrients across the membranes of their roots. In order to do this, the nutrients must be dissolved by water. The solution then bathes the roots, and proteins embedded in the root membranes can transport the nutrient into the cells. Once the cells have the nutrients, more water floods the cells. This mechanism in plants allows water and nutrients to flow all the way from the roots to the top leaves, even in the tallest trees. At the leaves, the plant transpires the water into the air, allowing the osmotic pressure to continue forcing nutrients and water up the leaves. This is all possible because water is an excellent solution which creates the solutions necessary for life.
Also Check: Which Founding Contributors To Psychology Helped Develop Behaviorism
What Does Washing And Drying A Solution Mean
After combining two solutions, a direction in a 1970s British chemistry book says to wash the new solution with a mixture of baking soda, table salt, and water. Then it says to dry it with anhydrous magnesium sulfate.
What does washing and drying mean? Do the chemicals get physically mixed together? Doesn’t that contaminate the solution?
This is standard for purifying substances.
To wash means to add your product solution to an aqueous solution to a separatory funnel. After shaking, you drain the lower layer . This process removes water soluble impurities. This is frequently repeated.
Drying is accomplished by taking the organic solution of your product and mixing it with a desiccant . This removes any water that was left from the washing in the above step. The desiccant may then be removed by filtration.
The solvent of this experiment should be non polar, and may be a hydrocarbon, or a chlorinated hydrocarbon. This organic phase may contain as impurities some acids and other polar substances soluble in water, that should be eliminated. This liquid is then mixed with an aqueous solution of soda $\ce$. Aqueous solutions and non polar substances are not miscible. But the soda reacts with the acidic impurities, producing a salt which is soluble into water. So, by stirring the two liquids, the impurities of the organic phase will pass into water, and are eliminated from the organic phase. This organic phase is said to have been “washed” by the soda solution.
Concentration Of A Solution

The concentration of a solution is the amount of solute present in a given quantity of it. In other words, the concentration of a solution is the mass of the solute in grams, which is present in 100 g of a solution. Depending upon the amount of solute present, it is called a dilute, concentrated or a saturated solution.
Different substances in a given solvent have different solubilities at the same temperature. The most common method for expressing the concentration of a solution is the percentage method. The concentration of the solutions refers to the percentage of solute present in the solutions. The percentage of solute can be expressed in terms of the following two quantities:
- Concentration of solutions in terms of mass of solute
If the solutions have solid solute dissolved in a liquid, then we consider the mass percentage of solute in calculating the concentration of the solutions. So, in the case of a solid solute dissolved in a liquid solvent. Mass by mass percentage of the solutions is given by the percentage of the mass of solute in 100 grams of solvent.
- Concentration by mass by volume percentage of a solution
Mass by volume percentage of a solute is the percentage of the mass of the solute present in the specific volume of the solvent. Depending upon the unit of the mass and volume, the mass by volume percentage of solute in solutions can have following units as gram/ml or gram/litre.
- Solubility of a Solute
You May Like: Define Span Linear Algebra
Princeton’s Wordnetrate This Definition:
solutionnoun
a homogeneous mixture of two or more substances frequently a liquid solution
“he used a solution of peroxide and water”
solution, answer, result, resolution, solventnoun
a statement that solves a problem or explains how to solve the problem
“they were trying to find a peaceful solution” “the answers were in the back of the book” “he computed the result to four decimal places”
solutionnoun
a method for solving a problem
“the easy solution is to look it up in the handbook”
solution, rootnoun
the set of values that give a true statement when substituted into an equation
solutionnoun
the successful action of solving a problem
“the solution took three hours”
Breaking Up The Solute
The first process that happens deals only with the solute, A, which requires breaking all intramolecular forces holding it together. This means the solute molecules separate from each other. The enthalpy of this process is called \. This since this is always an endothermic process , then \.
\ A } \nonumber \]
Also Check: Geometry Eoc Fsa Practice Test (calculator Portion)
What Happens When A Supersaturated Solution Is Cooled
The solid crystals in the hydrated crystals will dissolve into the bath, forming a supersaturated solution. When the solution for sodium thiosulfate is gradually cooled the super-saturated solution should remain liquid. Placing a small crystal in the over-saturated solution would make the liquid solid.
Put your understanding of this concept to test by answering a few MCQs. Click âStart Quizâ to begin!
Select the correct answer and click on the âFinishâ buttonCheck your score and answers at the end of the quiz
The Definition Of A Solution
Can there be more than one solute or solvent in a solution. Why? Why not? The largest component in the solution is called a solute and smallest a solvent. How is this possible when solutions contain more than 2 substances?
- $\begingroup$You can have as many solutes as it gets. Also there can be more of them in total then the amount of solvent present, so could you care less about semantics?$\endgroup$
- MithoronAug 19 ’16 at 1:21
- $\begingroup$Well no, I have an exam on this. Solutes are defined as the smallest component in the solution, so if there are multiple solutes, what are they called?$\endgroup$
Here is the solution defined by IUPAC:
- A liquid or solid phase containing more than one substance, when for convenience one substance, which is called the solvent, is treated differently from the other substances, which are called solutes. When, as is often but not necessarily the case, the sum of the mole fractions of solutes is small compared with unity, the solution is called a dilute solution. A superscript attached to the symbol for a property of a solution denotes the property in the limit of infinite dilution.
When the solution contains more than 3 components, one or more will be solvent.
For example, You can regard glass as solution, because it contains SiO2, Na2CO3, and many other components.
I think which is solvent is choose by human, usually the most part of the “solution” will be regard as solvent.
Read Also: How Do You Do Percent Error In Chemistry
The Relationship Between Molar Solutions And Molar Concentration
While they may sound similar, a molar solution is not the same as molar concentration. Molar concentration, also known as molarity, is the number of moles per liter of solution .
Molar concentration = mol of solute / L of solution
A molar solution, on the other hand, has 1 mol of solute contained in 1 liter of solution. In other words, a molar solution is simply a substance with a molarity of 1.
What Does Molar Solution Mean
A molar solution is defined as an aqueous solution that contains 1 mole of a compound dissolved in 1 liter of a solution. In other words, the solution has a concentration of 1 mol/L or a molarity of 1 . Physicists and chemists typically use this parameter to express concentrations of various substances.
Molar solutions and molarity measurements are frequently used in electrochemistry to quantify the concentration of ions in an electrolyte. The higher the concentration of a particular ion in a substance, the more aggressive it will likely be towards metals.
Molar solutions are also useful in predicting corrosion rates. For example, steel corrosion in a 1M hydrochloric acid solution can be assessed using weight loss and other electrochemical techniques. This information can then be used to perform calculations and evaluate steel corrosion in different situations.
Recommended Reading: Who Is Paris Jacksons Biological Father
Webster Dictionaryrate This Definition:
Solutionnoun
the act of separating the parts of any body, or the condition of undergoing a separation of parts disruption breach
Etymology:
Solutionnoun
the act of solving, or the state of being solved the disentanglement of any intricate problem or difficult question explanation clearing up — used especially in mathematics, either of the process of solving an equation or problem, or the result of the process
Etymology:
Solutionnoun
the state of being dissolved or disintegrated resolution disintegration
Etymology:
Solutionnoun
the act or process by which a body is absorbed into a liquid, and, remaining or becoming fluid, is diffused throughout the solvent also, the product reulting from such absorption
Etymology:
Solutionnoun
release deliverance discharge
Etymology:
Solutionnoun
the termination of a disease resolution
Etymology:
Solutionnoun
a crisis
Etymology:
Solutionnoun
a liquid medicine or preparation in which the solid ingredients are wholly soluble
Etymology:
Solution Definition In Chemistry

- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
A solution is a homogeneous mixture of two or more substances. A solution may exist in any phase.
A solution consists of a solute and a solvent. The solute is the substance that is dissolved in the solvent. The amount of solute that can be dissolved in solvent is called its solubility. For example, in a saline solution, salt is the solute dissolved in water as the solvent.
For solutions with components in the same phase, the substances present in lower concentration are solutes, while the substance present in highest abundance is the solvent. Using air as an example, oxygen and carbon dioxide gases are solutes, while nitrogen gas is the solvent.
Don’t Miss: Geometry Unit 4 Test Answer Key
What Is A Mixture
Mixtures are substances that consist of two or more types of matter. Air, soil, blood, etc. are different examples of mixtures. Based on the nature of the components and their distribution, mixtures are classified as homogeneous and heterogeneous mixtures.
- A mixture that has its components uniformly distributed is known as a homogeneous mixture.
- While if the distribution is non-uniform, the mixture is called a heterogeneous mixture.
A solution is a homogeneous mixture of two or more components. Lets learn more about solutions, its properties, how to find a concentration of solutions.
What Are Ten Examples Of Solutions That You Might Find In Your Home
A solution is a homogenous mixture that contains two or more substances. Solutions contain a solvent and a solute . Household solutions often appear in the form of substances dissolved into water, such as juice from concentrate.
Who are the experts?Our certified Educators are real professors, teachers, and scholars who use their academic expertise to tackle your toughest questions. Educators go through a rigorous application process, and every answer they submit is reviewed by our in-house editorial team.
Any substance dissolved in another substance is considered a solution. The solvent in most household substances is water. So, anything dissolved in water would make a solution. The dissolved substance is called the solute. You can dilute a solution by adding more solvent or…
Don’t Miss: Half Life Formula Chemistry
Why Are Supersaturated Solutions Unstable
Under appropriate conditions, solutions may often be formulated that contain a greater amount of solvent than the one required to form a saturated solution. Owing to the presence of the solute in a supersaturated solution in a concentration greater than the concentration of equilibrium, super-saturated solutions are unstable.
Effects Of Pressure On The Solubility Of Gases: Henrys Law
External pressure has very little effect on the solubility of liquids and solids. In contrast, the solubility of gases increases as the partial pressure of the gas above a solution increases. This point is illustrated in Figure 7.6, which shows the effect of increased pressure on the dynamic equilibrium that is established between the dissolved gas molecules in solution and the molecules in the gas phase above the solution. Because the concentration of molecules in the gas phase increases with increasing pressure, the concentration of dissolved gas molecules in the solution at equilibrium is also higher at higher pressures.
Figure 7.6 A Model Depicting Why the Solubility of a Gas Increases as the Partial Pressure Increases at Constant Temperature. When a gas comes in contact with a pure liquid, some of the gas molecules collide with the surface of the liquid and dissolve. When the concentration of dissolved gas molecules has increased so that the rate at which gas molecules escape into the gas phase is the same as the rate at which they dissolve, a dynamic equilibrium has been established, as depicted here. Increasing the pressure of the gas increases the number of molecules of gas per unit volume, which increases the rate at which gas molecules collide with the surface of the liquid and dissolve. As additional gas molecules dissolve at the higher pressure, the concentration of dissolved gas increases until a new dynamic equilibrium is established.
C = kP
Note the Pattern
Also Check: Eoc Fsa Warm Ups Algebra 1 Answers
What Is A Supersaturated Sugar Solution
According to the solubility of the substance a âsupersaturatedâ solution produces more dissolved content than it should. In the case of sugar, whose chemical name is âsucrose,â approximately 211 grams of water can dissolve in 100 millilitres. Solubility is temperature dependent more sugar dissolves in hot water than in cold.
Chemistry Glossary Definition Of Standard Solution
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
A standard solution is any chemical solution which has a precisely known concentration. Similarly, a solution of known concentration has been standardized. To prepare a standard solution, a known mass of solute is dissolved and the solution is diluted to a precise volume.
Standard solution concentration is usually expressed in terms of molarity or moles per liter . Not all substances are suitable solutes for standard solutions. The reagent must be stable, pure, and preferably of high molecular weight.
Also Check: How Many Subfields Of Psychology Are There
What Is Meant By Concentration Of A Solution
In an aqueous solution, two parts exist, namely solute and solvent. They are the two basic solution concentration terms that you need to know. We always need to keep an account of the amount of solute in the solution. The amount of solute in the solvent is what is called the concentration of a solution. In chemistry, we define concentration of solution as the amount of solute in a solvent. When a solution has more solute in it, we call it a concentrated solution. Whereas when the solution has more solvent in it, we call it a dilute solution. Now that you understand the concept of what is concentration of solution let’s move on to the different methods of expressing concentration.
The image shows a solution from the most dilute solution to the most concentrated solution.
Polar And Nonpolar Solutions
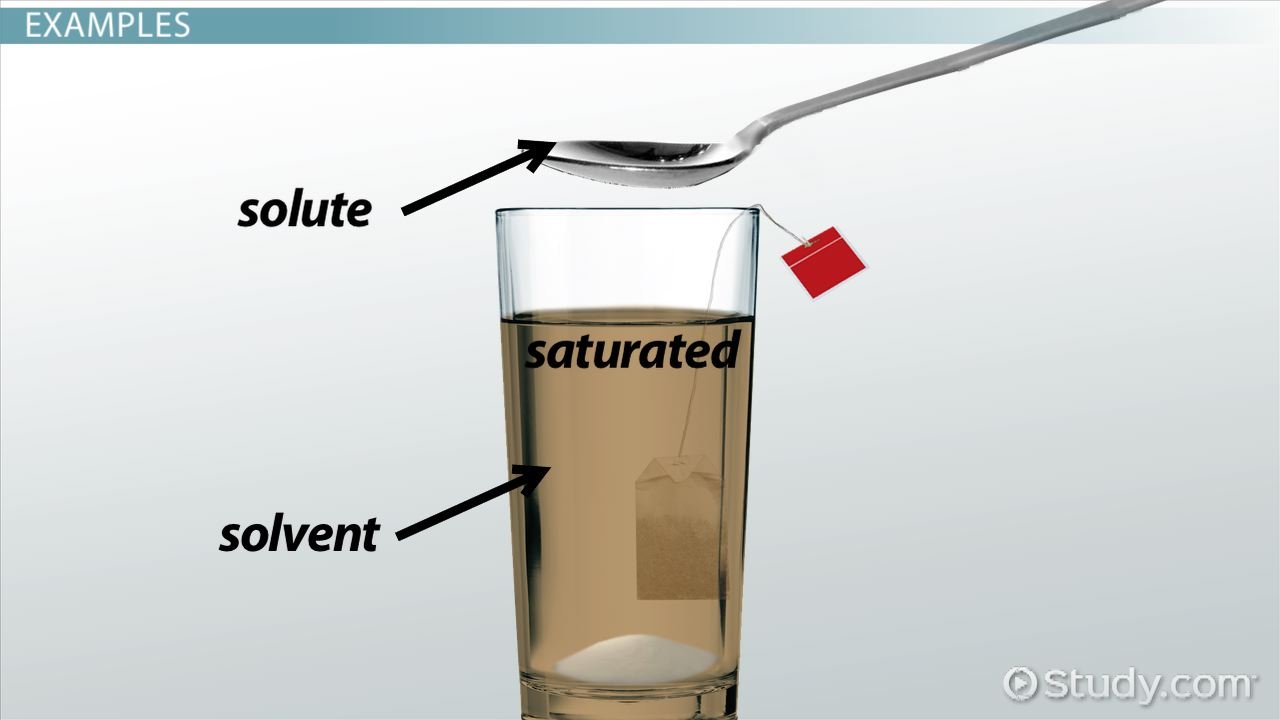
A polar solution is created when a polar solvent dissolves a polar solute. The opposing charges on the solvent molecules interact with the opposite charges on the solute molecules, which distributes them throughout the solvent. In a polar solution, the bonds are statically charged, in that they do not change. This is not the case of a nonpolar solution.
In a nonpolar solution, the same principle of opposite charges acting on each other causes the solvent to dissolve the solute. However, nonpolar molecules do not have static charges. Instead, the electrons sometimes group on the same side of the molecule. This negative area pushes the electrons on other molecules away, and creates positive areas of charge. These induced charges move throughout the solution, stirring it and moving the solute molecules about.
Recommended Reading: Holt Geometry Lesson 4.5 Practice B Answers