Which Electrons Count As Pi Electrons And Which Do Not
That seems straightforward enough. However, complications can arise when we have atoms in the ring which both participate in pi bonding and also have a lone pair. For example,
- how do we count electrons in the benzene anion or pyridine? Do we count the lone pair electrons as Pi electrons, giving a total of 8? Or do we ignore them?
- What about furan which has two lone pairs on oxgyen?
- What about pyrrole, with its lone pair on nitrogen, or imidazole, with two nitrogens?
In order to answer these questions, its important to remind ourselves of how p orbitals contribute to aromaticity in benzene.
In benzene, each p orbital is arranged at right angles to the plane of the ring. Each p orbital contains a single electron. We can verify the total number of pi electrons in benzene by counting the pi bonds: 3 pi bonds times two electrons = 6 pi electrons total.
Note that the C-H bonds are at 90° to the pi system. If there was a lone pair where the C-H bond is, then it wouldnt be able to interact with the pi system at all. Which brings us to.
Iupac Nomenclature Of Aromatic Compounds
Earlier, most of the compounds with the same structural formula were known by different names depending on the regions where they were synthesized. This naming system was very trivial since it raised a lot of confusion. Finally, a common naming system enlisting standard rules was set up by IUPAC for the naming of compounds. This method of naming is IUPAC naming or IUPAC nomenclature.
IUPAC nomenclature of aromatic hydrocarbons is explained below:
1. According to IUPAC nomenclature of substituted aromatic compounds, the substituent name is placed as a prefix to the name of aromatic compounds. For example, a benzene ring attached to a one-nitro group is named as nitrobenzene.
2. When more than one similar substituent group is present in the ring, they are labelled with the Greek numerical prefixes such as di, tri, tetra to denote the number of similar substituent groups attached to the ring. If two bromo- groups are attached to the adjacent carbon atoms of the benzene ring, it is named 1,2-dibromobenzene.
3. When different substituted groups are attached to the aromatic compounds, the substituent of the base compound is assigned number one and then the direction of numbering is chosen such that the next substituent gets the lowest number. Substituents are named in alphabetical order. For example: when chloro and nitro groups are attached to the benzene ring, we first locate the chloro group then nitro groups.
What Does N Mean In Iupac Naming
3.9/5nomenclaturenamingIUPAC nomenclaturen
Keeping this in view, what does N mean when naming amines?
3. The N– prefix. When it’s used: for amines and amides. What it means: The N signifies that the substitutent is connected to the nitrogen. Example: N-methyl butylamine, N,N-dimethylformamide.
Also, what is the meaning of N in N hexane? The letter n is used in front of hexane in order to differentiate the normal straight-chain hexane from its isomers.
In this way, what does the N in n propyl mean?
It consists of all four carbon atoms forming a chain and the rest of the molecule attaches at the first carbon. The n– stands for ‘normal’. In common names, the molecule would have n-butyl added to the molecule name.
What is lowercase n in chemistry?
n-, a lowercase prefix in chemistry denoting the straight-chain form of an open-chain compound in contrast to its branched isomer. N-, an uppercase prefix in chemistry denoting that the substituent is bonded to the nitrogen, as in amines.
Also Check: Do You Capitalize Bachelor’s Degree In Psychology
What Is Aromatic In Organic Chemistry
organic chemistryaromaticityaromatic
. Moreover, what does it mean to be aromatic?
1 : of, relating to, or having a smell or odor. 2 of an organic compound : characterized by increased chemical stability resulting from the delocalization of electrons in a ring system containing usually multiple conjugated double bonds compare alicyclic, aliphatic. aromatic.
Beside above, what is Huckel rule explain with example? Because a closed bond shell of electrons defines an aromatic system, you can use Hückels Rule to predict the aromaticity of a compound. For example, the benzene molecule, which has 3 bonds or 6 electrons, is aromatic.
One may also ask, what is aromaticity with example?
In organic chemistry, the term aromaticity is used to describe a cyclic, planar molecule with a ring of resonance bonds that exhibits more stability than other geometric or connective arrangements with the same set of atoms. For example benzene ring C6H6.
What does aromatic mean LGBT?
An aromantic is a person who experiences little or no romantic attraction to others. The aromantic attribute is usually considered to be innate and not a personal choice, just as the lack of sexual attraction is innate to asexuals.
Choose The Right Synonym For Aromatic
Adjective
odorous, fragrant, redolent, aromatic mean emitting and diffusing scent. odorous applies to whatever has a strong distinctive smell whether pleasant or unpleasant. odorous cheeses should be tightly wrappedfragrant applies to things with sweet or agreeable odors. a fragrant rose redolent applies usually to a place or thing impregnated with odors. the kitchen was redolent of garlic and tomatoes aromatic applies to things emitting pungent often fresh odors. an aromatic blend of tobaccos
Also Check: My Hrw Algebra 1
Scope Of Nomenclature For Organic Compounds
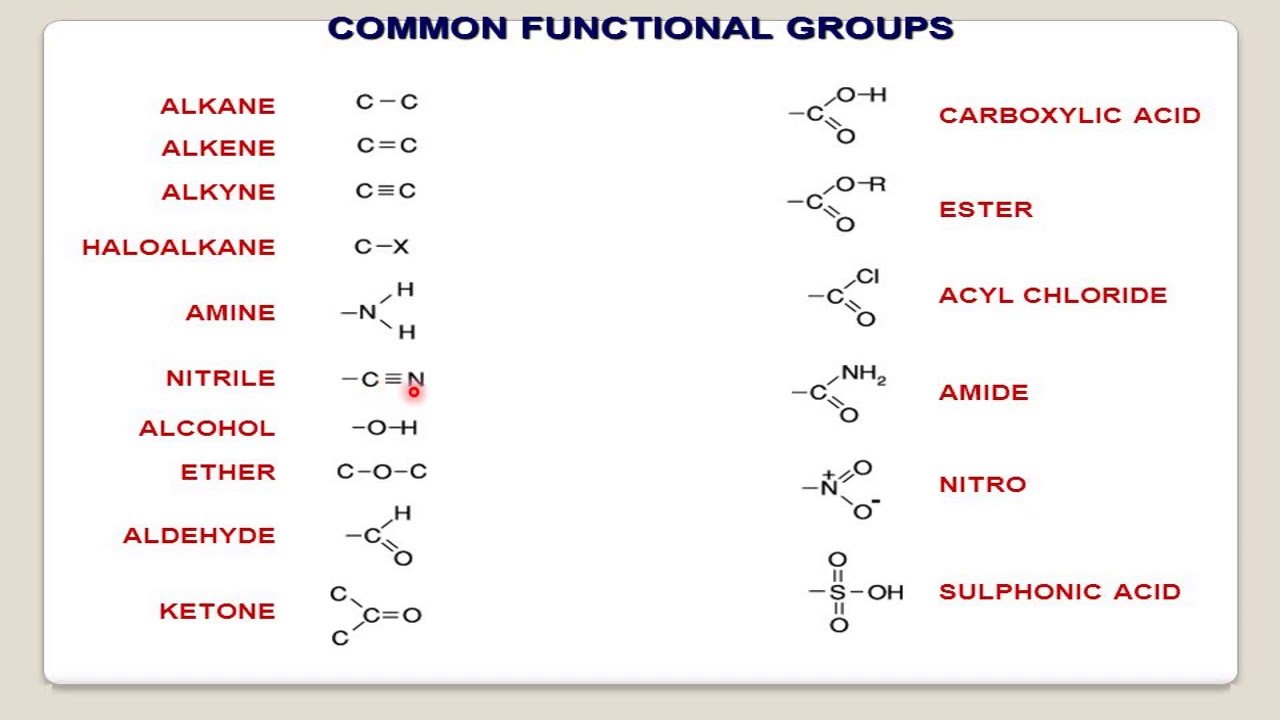
For nomenclature purposes all compounds containing carbon as the principal element to be organic compounds are qualified. Oxygen, hydrogen and nitrogen are the three elements usually associated with carbon to form the system of functional or characteristics groups. Other elements, among them the halogens and sulfur complete the basic core of elements found in organic compounds. Substitutive nomenclature was first applied to compounds containing this set of atoms. The success of this type of nomenclature was such that it was extended to all elements of Groups 14, 15, 16, 17 and in Group 13 to boron it could be extended to all elements of Group 13.
How Do You Know What Suffix To Use In Chemistry
When naming molecular compounds prefixes are used to dictate the number of a given element present in the compound. mono- indicates one, di- indicates two, tri- is three, tetra- is four, penta- is five, and hexa- is six, hepta- is seven, octo- is eight, nona- is nine, and deca is ten.
You May Like: Holt Geometry Lesson 4.5 Practice B Answers
What Is Iupac Nomenclature
IUPAC nomenclature of organic compounds refers to the systematic approach taken for the nomenclature of organic compounds as per the recommendation of the International Union of Pure and Applied Chemistry .
The necessity for such a systematic approach arose due to the sheer quantity of new discoveries of organic compounds which made the trivial nomenclature of organic compounds highly inconvenient.
However, the IUPAC nomenclature guidelines are not always followed by chemists since some compounds have very long and extremely tedious names as per the IUPAC nomenclature guidelines. These compounds are assigned more trivial names.
What Is The Iupac And What Does It Do
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
The IUPAC is the International Union of Pure and Applied Chemistry. It is an international scientific organization, not affiliated with any government. The IUPAC strives to advance chemistry, in part by setting global standards for names, symbols, and units. Nearly 1200 chemists are involved in IUPAC projects. Eight standing committees oversee the Union’s work in chemistry.
Don’t Miss: Geometry Basics Segment Addition Postulate Worksheet Answer Key
From The Structure Of Tetrodotoxin
Tetrodotoxin, Fig. 35 reproduced from , p. 73
Certain varieties of puffer fish, especially the tora fugu, or tiger puffer , and the closely related ma fugu¸ or common puffer , are highly prized as comestibles in Japan. The indulgence of the taste is fraught with some peril, since the livers and ovaries of the fish contain a powerful poison. The presence of this poison has been known though its effects since antiquity, but its labile nature and its extremely low concentration in its natural milieu made the isolation of the toxic principle extraordinarily difficult . . . Although nearly five hundred persons died from its effects in Japan during 1956-58, the toxic dose for man is not known if the physiologically absurd equation of men with mice be made, it may be anticipated that half a milligram of tetrodotoxin should be sufficient to deprive an average-sized man of his life.
Now, in a wholly imaginary experiment, . . .
The simplest manner in which the expression might be disabused of its unwanted carboxyl group was to . . .
At this point, however, the argument was simplified through the obtention of the almost ocular evidence provided by a complete three dimensional X-ray crystallographic analysis . . .
Components Of Systematic Substitutive Names
Locants indicate the position of substituents or other structural features. They are generally placed before the part of the name that indicates the corresponding structural feature. Three kinds of enclosing mark are used, in the nesting order , when it is necessary to indicate which parts of a name belong together.
Multiplicative prefixes are used when more than one fragment of a particular kind is present in a structure. Which kind of multiplicative prefix is used depends on the complexity of the corresponding fragment e.g. trichloro, but tris.
| hept |
a. Determine the principal characteristic group to be cited as the suffix .b. Determine the senior parent amongst those structural components attached to a principal characteristic group .c. Name the parent hydride and specify any unsaturation .d. Combine the name of the parent hydride with the suffix for the principal characteristic group . e. Identify the substituents and arrange the corresponding prefixes in alphabetical order.f. Insert multiplicative prefixes, without changing the already established order, and insert locants.g. Determine chirality centres and other stereogenic units, such as double bonds, and add stereodescriptors.
Don’t Miss: Eoc Fsa Warm Ups Algebra 1 Answers
International Union Of Pure And Applied Chemistry
IUPAC has been a member since 1922.
The International Union of Pure and Applied Chemistry , established in 1919, is the international body that represents chemistry and related sciences and technologies. IUPAC currently has 44 National Adhering Organizations and 21 Associate National Adhering Organizations.
IUPAC promotes continuing cooperation among the chemists of its Member Countries studies topics of international importance to chemistry requiring standardization or codification cooperates with other international organizations in topics of a chemical nature and contributes to the advancement and understanding of pure and applied chemistry in all aspects. IUPAC primarily pursues these objectives through the contributions of over 1000 volunteer chemists. Nearly 5000 chemists are enrolled in the Affiliate Member Programme, which provides a virtual channel of global communication.
IUPAC is recognized as the international authority on chemical nomenclature, terminology, symbols, units, atomic weights and related topics. Its reports and recommendations on these matters are often accepted as definitive and form the basis for drafting regulations relating to chemical manufacturing, international commerce, and matters relevant to food, health and the environment.
IUPAC maintains a professionally staffed Secretariat headed by an Executive Director in Research Triangle Park, North Carolina, USA.
- T: +33 1 45 25 03 29
- F: +33 1 42 88 94 31
What Does Iupac Stand For
What does IUPAC mean? This page is about the various possible meanings of the acronym, abbreviation, shorthand or slang term: IUPAC.
Filter by:
Popularity rank for the IUPAC initials by frequency of use:
Couldn’t find the full form or full meaning of IUPAC?
Maybe you were looking for one of these abbreviations:
Discuss these IUPAC abbreviations with the community:
Report Comment
We’re doing our best to make sure our content is useful, accurate and safe.If by any chance you spot an inappropriate comment while navigating through our website please use this form to let us know, and we’ll take care of it shortly.
Read Also: Segment Addition Postulate Practice
Example Of Iupac Nomenclature
Considering the following Example:
- There exist 9 carbon atoms on the straight chain and the 5th carbon atom consists of a substituent group which in turn has 3 carbon atoms in a chain.
- Furthermore, there the first and second carbons of this substituent chain have an additional CH group attached to them.
- In the nomenclature of this compound, the 9 membered carbon chain is identified as the parent chain and is numbered.
- The substituent chain attached to position 5 of the parent chain is 3 members long, with 2 methyl groups attached at positions 1 and 2.
- Thus the carbon chain substituent group on the parent chain can be called 1,2 dimethyl propane. The name for the substituent chain containing this compound would be 1,2 dimethyl propyl.
- Substituting this name on the parent chain, the IUPAC name of the compound in question is found to be: 5- nonane.
The International Union Of Pure And Applied Chemistry
The International Union of Pure and Applied Chemistry is the world authority on chemical nomenclature and terminology, including the naming of new elements in the periodic table on standardized methods for measurement and on atomic weights, and many other critically-evaluated data.
A neutral and objective scientific organization, IUPAC was established in 1919 by academic and industrial chemists who shared a common goal to unite a fragmented, global chemistry community for the advancement of the chemical sciences via collaboration and the free exchange of scientific information. Throughout its long history IUPAC has fulfilled that goal through the creation of a common language and the standardization of processes and procedures.
But IUPAC is about much more than nomenclature and the naming of elements. We are a leader in the provision of objective scientific expertise for the resolution of critical global issues that involve every aspect of chemistry, all of which have societal impact. Our scientific work is conducted largely through a formal project system, in which proposals from chemists around the world are peer-reviewed and, if meritorious, are approved and supported. In addition, IUPAC is involved in a wide range of diverse activities that ultimately impact both the chemical profession and society as a whole.
We are IUPAC International and Unique, advancing Pure and Applied Chemistry worldwide!
Also Check: Meaning Of Distance In Science
Corrosionpedia Explains Iupac Nomenclature
Since every chemical compound has an IUPAC naming convention, an unambiguous chemical formula can be created with ease. IUPAC nomenclature also exists for inorganic chemistry.
Basic rules are followed to create an IUPAC name for an organic compound:
- Identify the principal functional group and substituent. This can be done by selecting the class of the chemical compound. For example, an alcohol represents an R-OH group.
- Identify the longest continuous chain that contains this principal functional group. This defines the root name. The other groups that are attached to this root name are called substituents.
- Perform numbering for the principal functional group and substituents.
From Recent Advances In The Chemistry Of Natural Products
Vitamin B12 , sketch reproduced from , p. 520
. . . the crowded concatenation of six contiguous asymmetric centers embedded within the A/D moiety represents a formidable challenge.
Perhaps also this is the point at which I should emphasize explicitly the importance of the availability of the unnatural enantiomer. Much as had been our progress at this point, we were not unaware that we still had far to go, and that it might be either necessary or desirableas indeed it turned out to beto investigate a considerable number of alternatives for further advance. In these explorations we were able to utilize , confident that whatever new route we might establish through its study would be applicable to its counterpart of the natural series our experience has been such that this is just about the only kind of model study which we regard as wholly reliable! And in fact, although the reactions I shall describe in the sequel will be presented for compounds in the natural series, almost all of them were first discovered using the enantiomeric substances.
Woodward, with pencil in hand at the Woodward Research Institute in Basel, ca. 1965. His offices, either at Harvard or WRI, were often decorated with colorful flowers and cigarette-containing ashtrays. Photograph courtesy Chemical Heritage Foundation.
In the light of these considerations we were not surprised to find that our mesylate was indeed quite extraordinarily loath to undergo the desired transformation.
Also Check: Holt Geometry Workbook Answer Key
Order Of Precedence Of Group
When compounds contain more than one functional group, the order of precedence determines which groups are named with prefix or suffix forms. The table below shows common groups in decreasing order of precedence. The highest-precedence group takes the suffix, with all others taking the prefix form. However, double and triple bonds only take suffix form and are used with other suffixes.
Prefixed substituents are ordered alphabetically , e.g. chlorofluoromethane, not fluorochloromethane. If there are multiple functional groups of the same type, either prefixed or suffixed, the position numbers are ordered numerically The N position indicator for amines and amides comes before “1”, e.g., CH3CHCH
Popularity Rank By Frequency Of Use
How popular is IUPAC among other acronyms?
IUPAC also stands for:
Discuss this IUPAC abbreviation with the community:
Report Comment
We’re doing our best to make sure our content is useful, accurate and safe.If by any chance you spot an inappropriate comment while navigating through our website please use this form to let us know, and we’ll take care of it shortly.
You May Like: What Is Chunking In Psychology
How Do You Find The Iupac Name In Chemistry
IUPAC Rules for Alkane Nomenclature