Formulas And Names Of Binary Nonmetal
| Formula |
| Formula |
| Formula |
| Formula |
Rules For Naming Molecular Compounds:
Generally, the more electropositive atom is written first, followed by the more electronegative atom with an appropriate suffix. For example, \text_2\text can be called dihydrogen monoxide . Organic molecules do not follow this rule.
How Is An Mp3 Player Designed
In describing many technological items, it’s not enough to simply say what brand or model we have. We talk about details such as how much horsepower is âunder the hoodâ for a car or how fast the chip is for our computer. Even a simple device like an mp3 player has more than one size. We can get an 8 âââââââMB player, or a 16 MB player. Designation of the item often is incomplete without other information as to its capabilities.
Transition metals have more than one possibility for ion formation. In order to name these compounds correctly, we need to be able to indicate which ion is involved in any given compound.
Don’t Miss: Does Organic Chemistry Have Math
Formulas And Names Of Binary Metal
| Compound |
Binary Compounds Of Cations With Variable Charges Given Formula Write The Name Common Name System

A binary compound is one made of two different elements. There can be one of each element such as in CuCl or FeO. There can also be several of each element such as Fe2O3 or CuBr2.
This lesson shows you how to name binary compounds from the formula when a cation of variable charge is involved. The four formulas above are all examples of this type. Important point to remember: the cations involved in this lesson have variable charges. The anions involved have only one charge.
Antoine Laurent Lavoisier reformed chemistry in the late 1700’s with his publication of Méthode de nomenclature chimique in 1787 and Traitéélémentaire de Chimie in 1789. He is known as the “Father of Modern Chemistry.”
Two typical names of chemicals up to this point in history are “foliated earth of tartar” and “phlogisticated vitriolic acid.” There were hundreds of such names. One goal of the Méthode was to create chemical names based on the chemical composition.
Lavoisier’s solution, which will be studied in this lesson, was to use different suffixes to indicate differences in composition. Specifically, the use of “-ous” and “-ic” will be studied.
Here is what the IUPAC currently says about this naming system: “The following systems are in use but not recommended: The system of indicating valence by means of the suffixes -ous and -ic added to the root of the name of the cation may be retained for elements exhibiting not more than two valences.”
| Element |
Example #1: FeO
Read Also: Algebra Lineal Ejercicios Resueltos Numeros Complejos
The Stock Method Of Naming
An ionic compound is named first by its cation and then by its anion. The cation has the same name as its element. For example, \text^ is called the potassium ion, just as \text is called the potassium atom. The anion is named by taking the elemental name, removing the ending, and adding -ide. For example, \text^ is called fluoride, for the elemental name, fluorine. The -ine was removed and replaced with -ide. To name a compound, the cation name and the anion named are added together. For example, \text is also known as sodium fluoride.
If either the cation or the anion was a polyatomic ion, the polyatomic ion name is used in the name of the overall compound. The polyatomic ion name stays the same. For example, \text_3\text_2 is called calcium nitrate.
Naming Compounds Part 1 YouTube: This video explains how to name covalent and ionic compounds.
Presentation On Theme: Ionic Compounds Cont Using The Stock System Chemistry Joke A Neutron Sits Down At The Counter And Asks The Waitress How Much For A Coke The Waitress Presentation Transcript:
2 Ionic Compounds, Cont. Using The Stock System
3 Chemistry Joke A neutron sits down at the counter and asks the waitress, How much for a coke? The waitress replies For you, no charge!
4 Elements Weve Been Avoiding Metals that form more than one ion. Metals that form more than one ion. These include ALL metals not in Groups 1A or 2A except Ag, Zn,Cd, and Al. These include ALL metals not in Groups 1A or 2A except Ag, Zn,Cd, and Al. Ag Ag +1 Zn Zn +2 Cd Cd +2 Ag Ag +1 Zn Zn +2 Cd Cd +2 Formulas are written using the same 3-step Criss Cross method. Formulas are written using the same 3-step Criss Cross method. The difference comes in how they are named. The difference comes in how they are named.
5 For Example Iron forms both a 2+ ion and a 3+ ion. If we just name the ionic compound that iron forms with chlorine, iron chloride, is it iron +2 or iron +3? Iron 2+ or 3+ Would the formula be FeCl 2 or FeCl 3 ? Soto name compounds with these metals, we use the Stock Naming System. This system uses Roman numerals to indicate charge. , , , ,
6 Using the Stock System Chromium can form ions of +3 and +2. The Stock System uses Roman numerals to indicate charge, so we name the ion Chromium or Chromium . These Roman numerals will always refer to the charge of the metal before them. SoChromium is Cr 3+ AndChromium is Cr 2+ What is the ion symbol for Tin ? Sn 4+
8 Lets Name CuCl 2 and SnO 2 Copper Chloride and Tin Oxide
Recommended Reading: What Are Protecting Groups In Organic Chemistry
Common Names V Systematic Names
Many chemicals are so much a part of daily life that people know them by their familiar names. Ordinary cane sugar, for example, is more formally known as sucrose, but asking for it at the dinner table by that name will likely be a conversation stopper. Now imagine using its systematic name in the same context: Please pass the -D-glucopyranosyl—D-fructofuranoside! But saying sucrose would be quite appropriate if you needed to distinguish this particular sugar from the hundreds of other named sugars. And the only place you would come across a systematic name such as the rather unwieldy one mentioned above would be in scientific documentation in reference to a sugar that has no simple common name.
Many common chemical names have very old and intriguing origins, as the following two examples illustrate.
Most people associate the name ammonia with a gas with a pungent odor. While its systematic name, nitrogen trihydride , tells you its formula, what it will not tell you is the interesting history of its discovery. Smoke from burning camel dung condenses on cool surfaces to form a crystalline deposit, which the ancient Romans first noticed on the walls and ceiling of the temple that the Egyptians had built to the sun god Amun in Thebes. They named the material sal ammoniac, meaning salt of Amun. In 1774 Joseph Priestley found that heating sal ammoniac produced a gas with a pungent odor, which T. Bergman named ammonia eight years later.
Molecular Masses From Chemical Formulas
The molecular mass, or molecular weight of a compound is obtained by addingup the atomic masses of all of the atoms present within a unit of the substance.
For ionic compounds, the term formula mass or formula weight is used instead,since there aren’t really any molecules present.
The molecular/formula mass is numerically equal to the mass of one mole ofthe substance.
For example, the molecular weight of water would be obtained by the followingprocess:
Molecular mass of H2O = + = + amu = 18.02 amu
You May Like: How Many Subfields Of Psychology Are There
Comparison Of Enthalpy To Internal Energy
Pressure And Free Energy
You May Like: What Is Pi In Chemistry
General Practices In Naming
The general practice among chemists is to use the more common chemical names whenever it is practical to do so, especially in spoken or informal written communication. Many of the common names are known and used mainly by the scientific community. Chemical substances that are employed in the home, the arts, or in industry have acquired traditional or popular names that are still in wide use. Many, like sal ammoniac mentioned above, have fascinating stories behind their names.
Sulfuric acid: The historical name for sulfuric acid is oil of vitriol. Medieval European alchemists prepared it by roasting green vitriol in an iron retort. Its chemical formula is \text_2\text_4.
Naming Ionic Compounds With Transition Metals
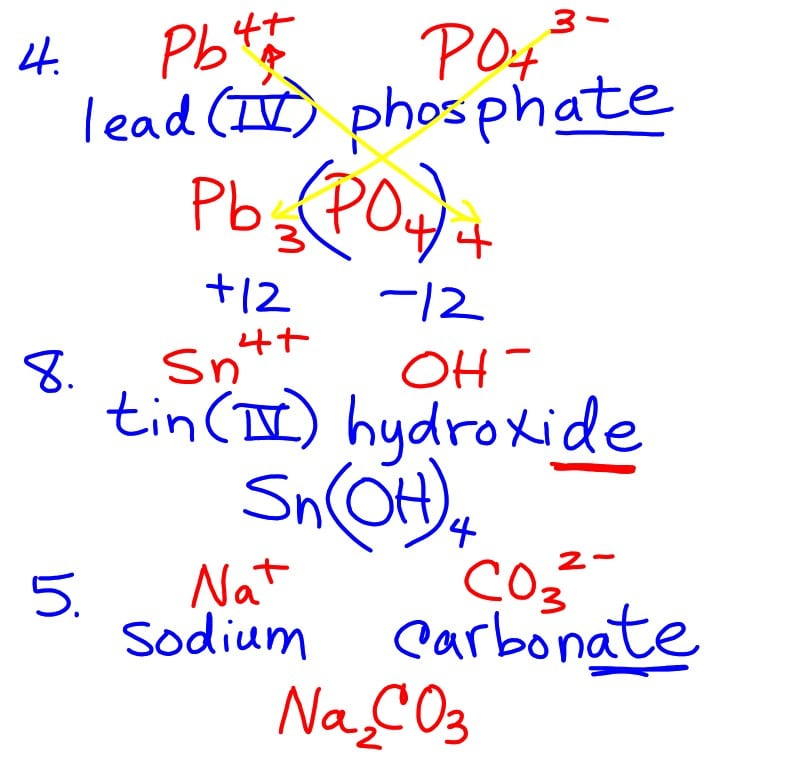
Transition metals make naming and formula writing a bit more challenging.The key to naming ionic compounds with transition metals is to determine the ionic charge on the metal and use roman numerals to indicate the charge on the transition metal.
Video: How to Name Ionic Compounds with Transition Metals
Naming Ionic Compounds with Transition Metals
- Write the name of transition metal as shown on the Periodic Table.
- Write the name and charge for the non-metal. If you have a polyatomic ion, use the Common Ion Table to find and write the formula and charge.
- Use the total charge on the non-metal find the charge on the transition metal.
- After the name for the metal, write its charge as a Roman Numeral in parentheses. Example: Iron chloride
Read Also: What Is Quantum Number In Chemistry
Naming Compounds Using The Stock System
Naming compounds that involve transition metal cations necessitates use of the Stock system. Consider the binary ionic compound \. To simply name this compound “iron chloride” would be incomplete, because iron is capable of forming two ions with different charges. The name of any iron-containing compound must reflect which iron ion is in the compound. In this case, the subscript in the formula indicates that there are three chloride ions, each with a \ charge. Therefore, the charge of the single iron ion must be \. The correct name of \ is iron chloride, with the cation charge written as the Roman numeral. Here are several other examples:
| Formula | |
|---|---|
| \ | tin oxide |
The first two examples are both oxides of copper . The ratio of copper ions to oxide ions determines the name. Since the oxide ion is \, the charges of the copper ion must be \ in the first formula and \ in the second formula. In the third formula, there is one tin ion for every two oxide ions. This means that the tin must carry a \ charge, making the name tin oxide.
Writing Formulas Of Ionic Compounds
Remember thePrime Directive in writing formulas:Ca2 ¹ CaOH2 !
Also Check: What Is The Electron Geometry Of So3